Lipid is an organic compound of biological origin that is made up of carbon, hydrogen, and oxygen. Lipid is esters of fatty acids and glycerol of higher aliphatic acids. It is insoluble in water and soluble in fat solvents like chloroform, ether, benzene, and alcohol.
Lipids comprise 0.5% of the living body. They are the naturally occurring substances in the body that are used for the storage of energy. Fatty type foods are good sources of lipids. Nuts, eggs, cheese, skimmed milk, cream butter, etc. all help the body`s lipid to do their job.
Properties of Lipids
Lipids are a group of organic compounds that are composed of fats and oils. They produce high energy and perform different functions within the human body. Some essential properties of lipids are listed below:
- Lipids are a complex heterogeneous group of molecules, which are mainly made up of hydrocarbon chains.
- They are oily and greasy substances which are found in the adipose tissue of the body.
- They play an important role in biological systems to form the cell membrane. In this case, the cell membrane acts as a mechanical barrier that divides the cell from the external environment.
- They are high energy-rich organic substances by which different life processes get energy.
- They are a class of compounds that are soluble in non-polar organic solvents like chloroform, acetone, alcohol, benzene, and insoluble in water.
- At room temperature, they are found as either liquids or non-crystalline solids.
- They do not contain any ionic charges.
- They have high proportions of saturated (solid triglycerols such as fats) and unsaturated (liquid triglycerols such as oils) fatty acids.
- They are odorless, colorless, and tasteless substances.
Some Examples of Lipids
Ghee, Butter, cheese, vegetable oil, cholesterol, and other steroids, phospholipids, waxes, and fat-soluble vitamins, etc.
You might also read: Vitamins : Classification, Functions and Deficiency Symptoms
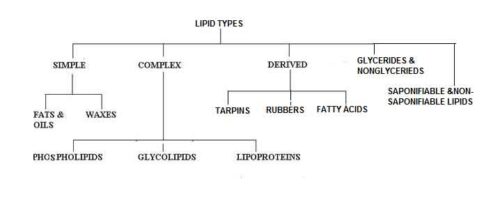
Classification of Lipids
Lipids are broadly classified into the following types:
- Simple lipids
- Complex lipids
- Glyceride & Non-glyceride lipids
- Derived lipids
- Saponifiable & non-saponifiable lipids
Simple lipids
Simple lipids are defined as those that on hydrolysis yield at most two types of primary product per mole. On hydrolysis, they give fatty acids and alcohol. Simple lipids are of the following types:
Triglycerides: A triglyceride is an ester derived from glycerol and three fatty acids. The main sources of triglycerides are foods such as butter, oils, and other fats you eat. If the body contains a high level of triglycerides which can cause heart diseases, such as coronary artery disease.
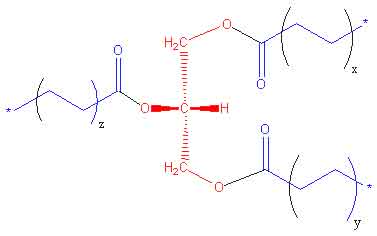
General Structure of Triglyceride
Some Factors that can raise your body triglyceride level include:
- Cigarette smoking
- Eating a lot of sugar
- Overweight
- Drinking of excessive alcohol
- Taking certain medicines
- Some genetic disorders
- Liver or kidney diseases
- Thyroid diseases
- Poorly controlled type 2 diabetes
The guidelines for triglyceride levels in the human body are:
|
Category |
Triglyceride Level |
|---|---|
|
Normal |
Less than 150mg/dL |
|
Borderline high |
150 to 199 mg/dL |
|
High |
200 to 499 mg/dL |
|
Very high |
500 mg/dL and above |
How will you control the high triglycerides level?
Following points should be maintained to lower your triglyceride levels with lifestyle changes:
- You should do regular physical activity
- You should spot smoking
- You should control your body weight
- Limiting the use of sugar and refined foods
- Restricting the use of alcohol
- Switching from saturated fats to healthier fats
Fats: Fats are essential substances that help to keep the skin healthy by using some vitamins. There are many types of fats in foods such as saturated, unsaturated, monounsaturated, polyunsaturated, and trans fats, etc.
Role of Fats
- Fats play the following several vital roles in the human body.
- Fats act as a dominant storage form of energy in the body.
- It helps to absorb certain nutrients and maintain body temperature.
- It acts as messengers and helps the proteins for performing their functions properly.
- Fats help to start many chemical reactions that involve growth, reproduction, immune function, and primary metabolism.
- Fat helps to absorb fat-soluble vitamins such as vitamin A, vitamin D, and vitamin E.
- Fats also help in the formation of cell materials and tissues.
Oils: The triglycerides produced unsaturated fatty acids are called oils. Unsaturated oils have a high melting point and are more like to be liquid at room temperature.
Waxes:Wax is an organic compound that may consist of 12-32 carbon atoms. It is derived from many different sources. Waxes are extremely hydrophobic and are completely insoluble in water. At room temperature, waxes are also solid.
Naturally occurring wax contains functional groups such as fatty acids, ketones, alcohols, aldehydes, and aromatic compounds, while synthetic waxes have aliphatic hydrocarbons with no functional groups.
In nature, many leaves, stems of plants, have wax, which creates coatings on leaves and stems and helps to inhibit the excessive water loss. Besides these, many animals bear wax-coated fur or feathers, which help to keep away water from the body.
Lipids are also classified into glycerides and non-glycerides lipids.
Glycerides: It is also called acylglycerols. They are glycerol-containing lipids and are formed collectively by glycerol and one to three fatty acids. They are the esters and occur naturally as fats and fatty oils. In this case, glycerol is a polyol compound which possesses three -OH groups, of which two serves as the primary and the other one as secondary. These -OH groups can be esterified with one, two, or three fatty acids and form monoglycerides, diglycerides, and triglycerides, respectively. It is not soluble in water and is less dense than water. The chemical properties of it mainly depend on its ester functions and the fatty acid chains that constitute them.
When you heat the glycerides or acylglycerols in an aqueous acidic medium, it becomes hydrolyzed and releases fatty acids and glycerol. When you also heat it in the presence of a strong base such as KOH or NaOH, it also releases glycerol and the corresponding fatty acid salts (soaps). This process is known as saponification.
Glycerides may be divided into the following two groups:
Neutral glycerides: They are non-ionic and non-polar. The neutral glyceride is produced by the esterification of glycerol with a fatty acid. The triglycerides are the most important neutral glycerides which form the major component of fat cells.
Phosphoglycerides: It is the most abundant membrane lipids which are originated from glycerol-3-phosphate. They have a polar region (the phosphoryl group) with non-polar fatty acid tails (the alkyl chain of the fatty acid).
Nonglyceride lipids: They are various types such as sphingolipids, steroids, and waxes.
Sphingolipids: It is a class of lipids that consists of an 18-carbon amino-alcohol backbone, sphingosine. These lipids were discovered in 1870 from the brain extracts. It was named after the mythological sphinx due to their enigmatic nature. It is the structural components of cellular membranes and is the second type of lipid, which constitutes the structural components of cellular membranes, particularly brain tissues and nerve cells. It is amphipathic which contains, a polar head group and two nonpolar fatty acid tails. These lipids play important roles in cell recognition and signal transduction. They are found in the membranes of both animal and plant cells. They also act as bioactive lipids and take part in a wide variety of biological functions, including cell propagation and cell death. They also maintain membrane functions, preserve lipoprotein structure and functions. They also prevent or promote many diseases such as atherosclerosis. They help to define the structural properties of membranes and lipoproteins. They play essential roles in cell-cell and cell–substratum interaction. They help to regulate cell differentiation and cell growth.
Steroids: Steroids are biologically active organic compounds that consist of four rings of carbon atoms arranged in a specific molecular configuration. They are hydrophobic and do not soluble in water. There are many naturally occurring steroids which are found in animals, plants, and fungi. In the human body, they act as hormones and play roles in absorption, reproduction, brain activity, and metabolism regulation. They are the important components of cell membranes, which can also alter the membrane fluidity.
Cholesterol: Cholesterol is a substance that has waxy like properties and is found only animal source foods. In the blood cells, different types of cholesterol are found, these are Triglycerides, LDL (Low-density lipoprotein or bad cholesterol ), IDL (intermediate-density lipoprotein), HDL( high-density lipoprotein) VLDL(very low-density lipoprotein), etc. Cholesterol is the precursor to many important steroid hormones such as progesterone, estrogen, and testosterone, which are produced by the endocrine gland (gonads). It plays an important role in the reproductive system. It also helps to synthesize the steroid hormones such as aldosterone, cortisol. In this case, aldosterone is used for osmoregulation, while cortisol helps in metabolism.
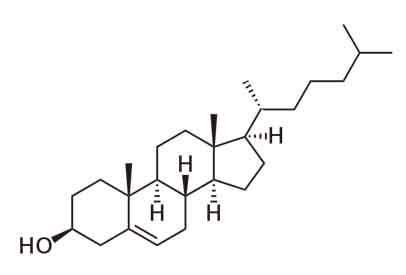
Structure of Cholesterol
Cholesterol is the most common steroid, which is a component of the phospholipid bilayer. It helps to play an important role in the structure of plasma membranes. It also involves in the cell to cell communication. It is the precursor to vitamin D and bile salt. In this case, bile salts help the cells to absorb fats.
Compound lipid
Compound lipids are those lipids that produce three or more primary products per mole when they are hydrolyzed. During hydrolysis, they give phosphate acid, different types of sugar, sphingosine, ethanolamine, and serine in addition to fatty acids and glycerol. They can be classified into the following main groups:
Phospholipid: When lipids contain two fatty acids, a glycerol unit, a phosphate group, and a polar molecule, then it is known as a phospholipid. They are the primary component of all cell membranes as they can form lipid bilayers. Industrially produced phospholipids are derived from soya, sunflower, rapeseed, bovine milk, fish eggs, chicken eggs, etc. Some notable phospholipids are lecithin, cephalins, phosphoinositides, etc.
Glycolipid: Glycolipids are any of a group of lipids that contain a carbohydrate group, commonly glucose or galactose. In this case, carbohydrate groups are attached by a glycosidic bond. Glycolipids are found in the cell membrane of eukaryotic cells. They perform essential functions in providing energy and act as receptors and markers for cellular recognition. They are also responsible for signal transduction and specific cellular contact.
Lipoprotein: A lipoprotein is a more complex substance than glycolipids, which contains both proteins and lipids. It contains a center core, which is made up of triacylglycerol molecules and cholesterol ester, while the surface of lipoprotein contains charged particles. In many organs, lipoproteins primarily involve transportation and delivery of cholesterol, fatty acids, and triacylglycerol to and from target cells.
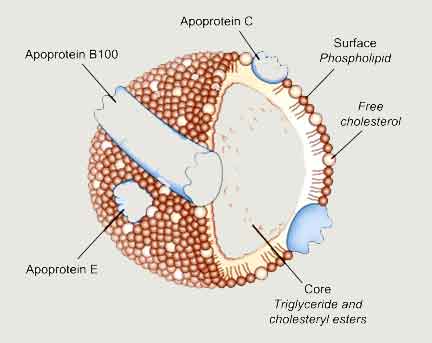
Lipoprotein
Lipoproteins are widely classified based on their density: The following table shows the basic characteristics of lipoproteins:
|
Types of Lipoprotein |
Density (g/mL) |
Primary Components |
Diameter (µm) |
|---|---|---|---|
|
Chylomicrons |
0.95 |
Dietary triacylglycerols (90%), phospholipids (6-12%), cholesterol (1-3%) and proteins (1-2%). |
75–1200 |
|
Very-low-density lipoprotein (VLDL) |
0.95–1.006 |
Endogenous triacylglycerols and cholesterol |
30–80 |
|
Intermediate-density lipoproteins(IDL) |
1.006–1.019 |
Triacylglycerols and cholesterol |
25 |
|
Low-density lipoproteins(LDL) |
1.019–1.063 |
Cholesterol. It is also informally known as bad chlolesterol. |
18–25 |
|
High-density lipoproteins |
1.063–1.210 |
Phospholipid and protein. It is also referred to as good cholesterol or healthy cholesterol because they can remove fat molecules from macrophages in the wall of arteries. |
5–12 |
Derived lipids
Hydrolysis products of simple and compound lipids are known as derived lipids. Some important derived lipids are:
- Terpins,
- Rubbers,
- Fatty acids,
Terpenes: Terpenes are one of the most important derived lipids. They are the components of the resin and essential oils of many types of plants and flowers. These molecules contain isoprene units (C5H8), and have the general formula (C5H8)n. Terpenes derived from oranges, lemons, other citrus, lavender, thyme, ceder wood, pine, and other plants more rarely by insects. Terpenes are used in the production of perfumes, insect repellants, cosmetics, cleaners, air fresheners, etc.
Rubbers: It is a derived lipid of high elastic solid substance with light cream or dark amber. It is polymerized by the drying and coagulation of the latex or milky juice of rubber trees and plants, especially of the Hevea brasiliensis (Pará rubber tree, sharinga tree), Palquium gutta, Ficus elastica (rubber fig), Castilla elastica (Panama rubber tree) and Taraxacum officinale ( common dandelion) species. Chemically, it is composed of 3000-6000 isoprene (C5H8) units. Rubber is also produced artificially. In the modern world, we use many rubber products for various purposes.
Fatty acids: They are either saturated and unsaturated carboxylic acids or organic acids, often with long-chain aliphatic tails. In general, most naturally occurring fatty acids have an even number of carbon atoms that are un-branched. Triglycerides are the main source of fatty acids in the diet, which is generically known as fats. Triglyceride is an ester that is derived from glycerol and three fatty acids. In the human body, fats make an important part of the diet, and it contributes about 45% of energy intake in many countries.
Saturated Fatty Acids: Saturated fatty acids are essential to nutrition, which contains straight-chain organic acids and an even number of carbon atoms. The term saturated demonstrates that the most extreme possible number of hydrogen atoms is attached to every carbon in the molecules. The chemical formula of saturated fatty acid is CH3(CH2)nCOOH. Saturated fats have a higher melting point and are more likely to be solid at room temperature (200c) due to their straight rod-like shape.
They have the ability to promote levels of blood lipid in the human body. They are originated from both plant oils and animal fats. There are many sources of saturated fatty acids in nature, including meat fat, butterfat, palm oil, palm kernel oil, and coconut oil.
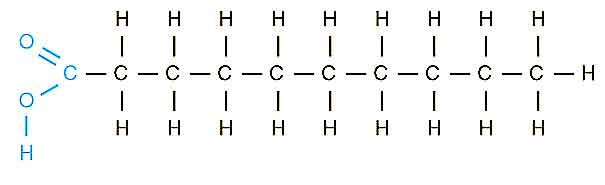
Structure of Saturated Fatty Acid
It is recommended that saturated fatty acid intake should be kept low. In Canada and the United States, the recent study showed that dietary recommendations of saturated fatty acids intake should be less than 10% of total caloric intake.
Some Important Saturated Fatty Acids
|
Common name and Chemical Formula |
Carbon numbers in chain |
Melting point |
Sources |
|---|---|---|---|
|
Lauric acid (C12H24O2) |
12 |
43.2 °C |
Palm kernel oil, nutmeg (Myristica fragrans) |
|
Myristic acid(C14H28O2) |
14 |
54.4 °C |
Palm kernel oil, nutmeg (Myristica fragrans) |
|
Palmitic acid (C16H32O2) |
16 |
62.9 °C |
Animal lipids, olive oil |
|
Stearic acid(C18H36O2) |
18 |
69.3 °C |
Animal lipids, cocoa butter |
|
Behenic acid(C22H44O2) |
22 |
80 °C |
Radish oil, brain tissue |
|
Lignoceric acid(C24H48O2) |
24 |
84.2 °C |
Carnauba wax, brain tissue |
Unsaturated fatty acids: If the fatty acids contain more than one double bond, then they are known as unsaturated fatty acids. They are less stable than saturated fatty acids. The term unsaturated demonstrates that the fewer than extreme possible numbers of hydrogen atoms are attached to every carbon in the molecules. The number of double bonds is denoted by the generic name. In this case, monounsaturated fatty acids for molecules are attached with one double bond, while polyunsaturated for molecules are bonded with two or more double bonds.
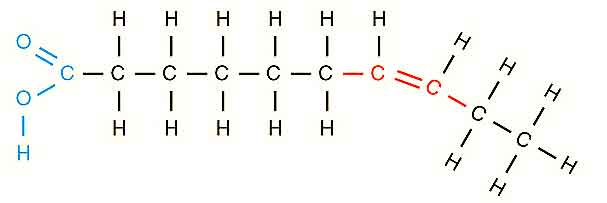
Structure of Unsaturated Fatty Acid
Naturally Occurring Unsaturated Fatty Acids
|
Common Name |
Molecular Formula |
Sources |
Melting Point (°C) |
|---|---|---|---|
|
Palmitoleic |
C16H30O2 |
Marine algae, Pine oil |
0.5 |
|
Oleic |
C18H34O2 |
Animal tissues, Olive oil |
13 |
|
Vaccenic |
C18H34O2 |
Formed by bacteria |
43 |
|
Linoleic |
C18H32O2 |
Plants and animals |
−5 |
|
Arachidonic |
C20H32O2 |
Liver, Brain tissue |
-50 |
|
Erucic |
C22H42O2 |
Mustard seed oil |
39 |
|
Nervonic |
C24H46O2 |
Sharks, Brain tissue |
– |
Based on reaction with NaOH/KOH lipids are also classified into the following two types:
Saponifiable lipids: A saponifiable lipid contains one or more ester functional groups that can be broken down under basic conditions by hydrolysis. These include triglycerides, phospholipids, glycolipids, sphingolipids, and waxes.
Non-saponifiable lipid: A non-saponifiable lipid does not contain an ester functional group, and that cannot be broken down into smaller molecules under basic conditions by hydrolysis. These lipids include prostoglandens, steroids, and terpenes.
Some Important Functions of Lipids in Living Organisms
Lipids are vitally important in living organisms. They have many functions, some of which are listed below:
- Formation of cell membranes: Phospholipids act as building blocks of the biological cell membranes in virtually all organisms.
- Storage of energy: Lipids are the primary energy sources of many organisms. They are stored in adipose tissue for later use and provide major parts of calories. The structure of lipids allows more energy to be stored in less space. On average, lipids provide 9.0 kcal/g energy. According to many Nutrition Organizations, lipids contribute up to 30% of the total daily energy intake.
- Hormones: Lipids form the base from which hormones are built. Many hormones are lipids like steroid hormones, such as estrogens, androgens, and cortisol, which are formed from cholesterol, which is essential during embryogenesis. Plants and animals use hormones to perform numerous important functions like regulating stress responses, sugar levels, and energy processing, as well as the production of sex cells.
- Absorbing vitamins: Some lipids are essential nutrients which contain necessary fat-soluble vitamins (vitamins A, D, E, and K) such are carried out of the intestines by the aid of lipids and stored in fatty tissues. In this case, vitamin A is necessary for vision, vitamin D for calcium metabolism which is present in fats and oils of animals, vitamin E helps to prevent autoxidation of unsaturated lipids and is present in vegetable oils while vitamin k is very important for normal clotting of blood which is present in green leaves.
- Protection: Many organisms use lipids for protecting their surfaces, which are exposed to the elements. Plants have waxy coatings on their leaves to prevent drying out, much like the oils found on skin, hair, and nails of mammals.
- Buoyancy: In aquatic mammals, the fat is less dense than water, so it acts as a buoyancy aid.
- Insulating the body: The human body contains a subcutaneous layer of fat that insulates the body and helps to maintain the temperature of the body. In nerve fibers, the myelin sheath also contains lipids that act as electrical insulators.
- Fluidity and permeability of cell membranes: Cholesterol regulates membrane fluidity over a broader range of temperatures. It also maintains the permeability of the plasma membrane.
- Transporter: Phospholipids play an essential role in the transport of fat between gut and liver in mammalian digestion.
- Coloration: Some lipid derivatives such as carotene, xanthophylls, chlorophylls, etc. are responsible for the coloration of plants.
- Odor: Terpenes produced perfumes in plants.
- Photosynthesis: Glycolipids help in the photosynthesis process in plants.
- Some lipids affect the texture and flavor of food and make food palatability.
- Many lipids such as ceramides, diacylglycerol, sphingosine, etc. act as regulators of intracellular processes.
- In the digestive tract, lipids facilitate the digestive process to depress gastric secretion. It also stimulates biliary and pancreatic flow.
- In many organisms, some lipids act as pheromones and are secreted into the external environment, which attracts or repels other organisms.
- In animals, some lipids are secreted from the epidermis, and they are involved in maintaining the water barrier.
Lipid Profile (mg/ID) Chart for humans
|
Interpretation |
TG |
LDL |
HDL |
Total cholesterol (TC) |
|---|---|---|---|---|
|
Normal range |
Less than 150 |
Below 100 |
145 |
Less than 200 |
|
Bordered line |
150-199 |
130-159 |
90-145 |
200-239 |
|
High risk |
200-499 |
160-189 |
Less than 90 |
240 and above |
|
Very high risk |
500 or above |
190 and above |
Less than 40 |
240 and above |
Concluding Remarks
Lipids are the naturally occurring substances that comprise 0.5% of the living body. They are used for storing energy, signaling, and acting as structural components of the cell membranes. During the growth of the body, lipids are used as “bricks” for the construction of biological membranes. They also play a significant role in the maintenance and repairing of cell membranes. At the present time, lipids are utilized in food industries, in the cosmetic as well as in nanotechnology.

