DNA is also known as deoxyribonucleic acid. German biochemist Frederich Miescher first observed DNA in 1869. He termed the material nuclein, which he isolated from pus cells that he collected from bandages discarded by a nearby clinic. Frederick Griffith realized that DNA might hold genetic information in 1928. Watson, Francis Crick, Maurice Wilkins, and Rosalind Franklin figured out the double helix structure of DNA in 1953. For their discoveries, Watson, Crick, and Wilkins rewarded the Nobel Prize in Medicine in 1962.
DNA contains double-stranded helical molecule. It is made up of purine and pyrimidine bases. In this case, purine and pyrimidine bases are attached pairwise by hydrogen bonds while sugar and phosphate make the longitudinal backbone of DNA.
DNA is the main chemical structure of chromosomes of eukaryotic cells. It is also found in mitochondria and chloroplast in less quantity of eukaryotic cells, the cytoplasm of prokaryotic cells, and in some viruses. In eukaryotic cells, it remains combined with protein to form nucleoprotein.
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are known as nucleic acid which are found in all living organisms. They are large molecules called macromolecules or polymers. They consist of repeating structural subunits monomer or nucleotides. Nucleotides are the building blocks of DNA, which consists of three parts: a pentose sugar (5-carbon sugar), a phosphate group, and a nitrogenous base.
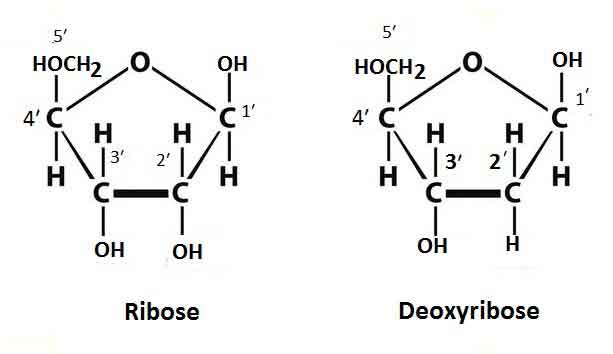
Pentose Sugar
It is a monosaccharide that contains five carbons. Pentose sugars are of two types, such as ribose (in RNA) and deoxyribose (in DNA). DNA contains 2-β-D type deoxyribose pentose sugar in which an oxygen atom is lacking in carbon two positions of pentose structure.
Phosphoric Acid
A phosphate group is attached to carbon at 3′ of one pentose sugar and carbon atom 5′ of another pentose sugar. In this case, the phosphate group of one nucleotide links covalently with the sugar molecule of the next nucleotide. In this way, they form a long polymer of nucleotide monomers.
Nitrogenous Bases
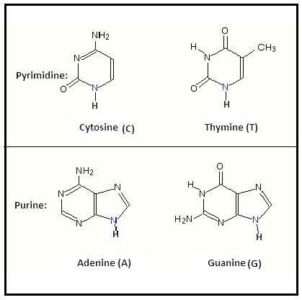
Four types of nitrogenous bases are found in DNA. These are Adenine (A), guanine (G), cytosine (C), and thymine (T). Among them, Adenine (A), guanine (G) are collectively called purine bases, while cytosine (C) and thymine (T) are called pyrimidine bases. Here pyrimidine bases (C4H4N2) are single-ringed compounds, and purine bases (C5H4N4) are double fused ring compounds.
Nucleotide
The nucleotide is a compound that is composed of three parts: a 5′ carbon pentose sugar molecule, nitrogenous base, and phosphate group. In this case, pentose sugar can be either ribose or a deoxyribose. Ribonucleotides or ribotides are the ribose containing nucleotide, while deoxyribonucleotides or deoxyribotides are the deoxyribose containing nucleotides.
Nitrogenous base is composed of purine and pyrimidine. In this case, purines are adenine and guanine while pyrimidines are cytosine, uracil and thymine.
Nucleotide = Sugar + Base + Phosphate
The Biological Functions of Nucleotides
- Nucleotide makes up the building blocks of life.
- It forms many different molecules that perform to make life possible.
- It acts as storage of genetic information, as part of RNA or DNA.
- It makes cellular communication.
- It helps in co-enzyme catalysis.
- It acts as messengers and energy moving molecules.
- In living organisms, it makes up the genetic material.
- It is involved in the synthesis of the polysaccharide.
- It also performs cell signaling, metabolism, and enzyme reactions.
Nucleoside
A nucleoside is made up of a pentose sugar molecule and a nitrogenous base. It does not contain any phosphate group. A nitrogenous base is covalently attached to a pentose sugar, which can be either ribose or deoxyribose. Ribose containing nucleosides are called ribonucleosides or ribosides, while deoxyribose containing nucleosides are called deoxyribonucleosides or deoxyribosides. In the case of nucleoside, nitrogenous bases and the pentose sugars are the same as in the nucleotide.
Nucleoside = Sugar + Nitrogenous base
Examples of nucleosides: Adenosine, thymidine, uridine, guanosine, cytidine etc.
Functions of Nucleosides
- It is a structural subunit of nucleic acids.
- It is heredity controlling components of all living cells.
The following table shows the examples of nucleosides and nucleotides with corresponding nitrogenous bases.
|
Nucleic Acid |
Nitrogen Base |
Nucleoside Sugar + Base |
Nucleotide Sugar+Base+Phosphate |
|---|---|---|---|
|
RNA |
Adenine(A) |
Adenosine |
Adenosine monophosphate(AMP) |
|
RNA |
Guanine(G) |
Guanosine |
Guanosine monophosphate(GMP) |
|
RNA |
Cytosine(C) |
Cytidine |
Cytidine monophosphate(CMP) |
|
RNA |
Uracil (U) |
Uridine |
Uridine monophosphate(UMP) |
|
DNA |
Adenine(A) |
Deoxyadenosine |
Deoxyadenosine monophosphate(dAMP) |
|
DNA |
Guanine(G) |
Deoxyguanosine |
Deoxyguanosine monophosphate (dGMP) |
|
DNA |
Cytosine(C) |
Deoxycytidine |
Deoxycytidine monophosphate(dCMP) |
|
DNA |
Thymine(T) |
Deoxythymidine |
Deoxythymidine monophosphate (dTMP) |
Structure of DNA
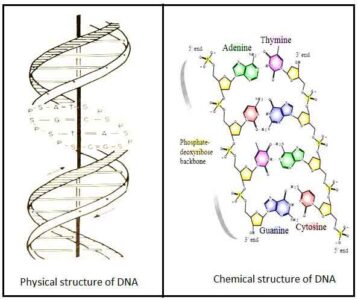
- Wilkin, Franklin, Watson, and Crick proposed a model for the DNA structure in 1953, based on the X-ray diffraction data.
- It is composed of two anti-parallel polynucleotide chains (strands) that form a double helix around a central axis. It looks like a ladder.
- Deoxyribose and phosphate groups alternately make the backbone of each stand or polynucleotide chain.
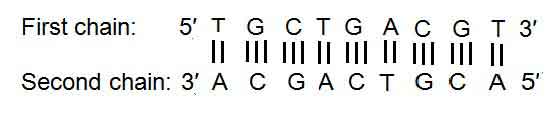
- The two strands are inter-twisted in a clockwise direction. In the two antiparallel strands, one strand runs 5′ to 3′ direction while the other runs 3′ to 5′ direction.
- The two strands are linked together by hydrogen bonds established between the pairs of the purine or pyrimidine bases.
- According to base-pairing rules, nitrogenous bases are linked together to make the double-stranded DNA molecule.
- Adenine always connected with thymine by two hydrogen bonds (A=T), and Guanine is connected with cytosine by three hydrogen bonds (G≡C).
- The helix makes one complete turn in every 340 in just over ten nucleotide pairs and has a diameter of about 20Å.
- Along one polynucleotide chain the axial sequence of bases may vary considerably, but the sequence must be complementary in the other chain. These sequences are given away in the following examples
Functions of DNA
- DNA is necessary for the production of proteins.
- It helps to regulate the metabolism and reproduction of the cell.
- It holds all of the genetic information.
- DNA produces characteristics of an individual and species.
- DNA plays a vital role in the replication of DNA hence increase in the number of chromosomes and cells.
- It helps the formation of RNA.
- DNA helps in the exchange of genetic information from parents to progeny.
Difference between Nucleoside and Nucleotide
|
Nucleoside |
Nucleotide |
|---|---|
|
It is made up of a pentose sugar and nitrogenous base. |
It is made up of pentose sugar, a phosphate group and a nitrogenous base. |
|
It is the precursor of nucleotide. |
It makes the precursor of deoxyribose nucleic acid, ribose nucleic acid and polynucleotides. |
|
Rich nucleosides` diet makes the optimum health. |
It helps to transfer transduction signal. |
|
There are several nucleoside analogues which are used in therapeutic drugs such as antiviral or anticancer agents to prevent viral replication in infected cells. |
Locked nucleic acid (LNA), Peptide nucleic acid (PNA), etc are the analogous for the sugar backbone in RNA which regulate the gene expression. |
|
Examples: Adenosine, thymidine, uridine, guanosine, cytidine, etc. |
Examples: AMP(Adenine monophosphate), ADP (adenine diphosphate), and ATP (adenine triphosphate), etc. |
Difference between Purine and Pyrimidine
|
Purines |
Pyrimidines |
|---|---|
|
Four nitrogen atoms and two hydrogen carbon rings makes the purine. |
In this case, two nitrogen atoms and one hydrogen-carbon ring makes the pyrimidine. |
|
Nucleonbases are adenine and guanine. |
Nucleonbases are Cytosine, thymine, and uracil. |
|
It is bigger in size. |
It is smaller in size. |
|
Melting Point: 417 °F (214 °C) . |
Melting point: 68 to 72 °F (20 to 22 °C). |
|
It`s chemical formula is C5H4N4. |
It`s chemical formula is C4H4N2. |
|
It is biosynthesized in liver. |
It is biosynthesized in various tissues. |
|
Catabolism Product of purine is uric acid (C5H4N4O3). |
Catabolism Products are ammonia (NH3) and carbon dioxide (CO2). |
|
It`s molar mass is 120.11 g mol−1. |
It`s molar mass is 80.088 g mol-1. |
|
It produces DNA and RNA. It is used in storage of energy. It synthesizes protein and starch. It can perform cell signaling. It also helps in enzyme regulation. |
It produces DNA and RNA. It is used in storage of energy. It synthesizes protein and starch. It can perform cell signaling. It also helps in enzyme regulation. |
Concluding Remarks
DNA or Deoxyribonucleic acid is the double-stranded helical molecule that contains genetic information. Swiss physician Friedrich Miescher first isolated the DNA molecule from the pus cells of discarded surgical bandages in 1869. Watson, Francis Crick, Maurice Wilkins, and Rosalind Franklin figured out the double helix structure of DNA in 1953. Generally, DNA occurs as linear chromosomes and circular chromosomes in eukaryotic and prokaryotic cells, respectively. Each genome is made by the set of chromosomes in a cell. There are approximately three billion base pairs of DNA in the human genome, which is arranged into 23 pairs of chromosomes. DNA carries and transmits the genetic information which is achieved via complementary base pairing.