Strongyloides stercoralis is a nematode parasite which belongs to the family Strongylidae under order Rhabditida. It is a human pathogenic parasite which causes disease, known as strongyloidiasis. The parasite is also known as threadworm causing infection with fecal contamination of soil or water. It is rare in developed countries while in the developing countries, it is more invasive in rural areas than urban areas.
Systematic Position
- Phylum: Nematoda
- Class: Chromadorea
- Order: Rhabditida
- Family: Strongylidae
- Genus: Strongyloides
- Species: Strongyloides stercularis
Morphology
The worm has both parasitic and free-living existence. The females are ovo-viviparous. The parasitic females live in the mucous membrane of the small intestine of human, especially in the duodenum and jejunum.
The oesophagous is a long cylindrical muscular tube with a posterior bulb containing valves and a prebulbar swelling. The females are considered to be parthenogenetic.
The males grow up to 0.9 mm (0.04 inch) in length while the females can grow up to 2.5 mm (0.10 inch) in length. The males have spicules and gubernaculum.

Larva: There are two types of larvae, Rhabditiform larvae and Filariform larvae. As soon as the eggs containing rhabditiform larvae are laid, the rhabditiform larvae start hatching. Hence the larvae and not the eggs are found in stool.
Rhabditiform larvae: It is the first stage larva which develops within the eggs in the gravid females. It immediately hatches out of the eggs in the mucosa of small intestine. It is an active larva which measures 200-300 µm in length. It has double bulb oesophagous with short mouth. It also contains inconspicuous genital primordium.
Filariform larva: The filariform larvae are infective stage. They enter the human host through the skin like ancylostoma. It is long and slender with 630 µm in length and 10 µm in breadth. The mouth is short with long cylindrical oesophagous.
Life Cycle
The worm passes its life cycle in human and a change of host is not essential as it undergoes autoinfection (hyperinfection). No intermediate host is required. Life cycle may be repeated by:
1. Rhabdititorm larvae voided with faeces. This may follow:
Direct Life Cycle
Parasitic females live in the intestine of human. Rhabditiform larvae pass out in the stool. They are metamorphosed into second-stage rhabditiform larva and then to form infective filariform larva within 3-4 days. The filariform larvae enter into the skin of bare-foot man. They are carried to the right side of the heart and then in pulmonary capillaries. They enter the lung alveoli. Then they migrate to the bronchi, trachea, larynx and epiglottis to pharynx and are swallowed. Reaching duodenum and jejunum, they develop into parasitic females and possibly males. The parasitic females then burrow into the mucous membrane and start to oviposit in the tissues.
Indirect Life Cycle (Free-living Life Cycle)
Rhabditiform larvae are voided with the stool. They develop into free-living adult males and females in the soil usually in moist tropical climates. The rhabditiform larva matures within 24-30 hours. After mating, the whole life cycle of egg, larvae, and adult can occur in soil. After several free-living cycles these metamorphose into infective filariform larvae within 3-4 days. They penetrate the skin. In this case, nearly 30 filariform larvae produce from each pair of rhabditiform larvae.
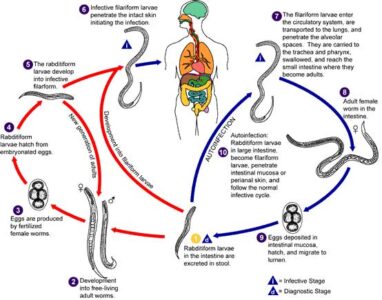
2. Autoinfection (Hyperinfection) Cycle
Rhabditiform larvae metamorphose into filariform larvae in the intestinal lumen which then penetrate the intestinal mucosa causing internal autoinfection or perineal skin causing external infection and enter the circulation. The females sometimes may enter the epithelium of bronchi and trachea where they oviposit. In this ways, the continuous infection occurs.
Pathogenesis
Infection with Stongyloides stercolaris is called stronguloidiasis. Many animal species are present. Mainly animal species causes cutaneous larva currens and larva migrans in human.
1. Skin: Skin penetration causes itching and red bloches at the site of entry of infective filariform larvae.
2. Intestinal: Mild inflammatory response when light infection. Ulceration and sloughing of the mucous occurs with heavy infection.
3. Pulmonary: Bronchopneumonia and haemorrhages in the lung alveoli, often associated with eosinophil infiltration. The changes occur during migration of filariform larvae.
4. Hyperinfection Syndrome: It develops due to massive strongyloidiasis in immuno-suppressive or immunodeficiency states. There is loss of defense against autoinfection. There may be systemic spread of larvae in all tissues of the body. The condition may be fetal.
5. Blood Changes: A marked eosinophilia occurs during the invasive stage.
Clinical Features
Light infections are asymptomatic.
1. Skin Symptoms: An uticarial rash at the site of entry of infective filariform larvae.
Larva currens, a serpiginous, migratory, raised utricarial eruptions usually on the buttocks, perineum or thighs is pathogenomic. Nonspecific maculopapular or utricarial eruptions may occur. This may also occur in patients with autoinfection. The lesions may advance as rapidly as 10 cm/hr along the course of larval migration.
2. Pulmonary Symptoms: Absent in most cases. In heavy infections during migration, the organisms may cause Loffler`s pneumonia and haemorrhages in the lung alveoli, often associated with eosinophil infiltration. Occasionally larvae mature in the bronchial submucosa and produce chronic bronchitis and asthma.
3. Intestinal Symptoms: Cause a burning mid-epigastric pain and tenderness accompanied by nausea and vomiting. Diarrhea and constipation may alternate. In heavy, chronic infections, malabsorption and protein-losing enterophathy may cause anemia, weight loss and cachexia. Dysentery may occur.
4. Hyper-infection Syndrome: The condition may be fatal even with treatment. The hyperinfection syndrome can result in disseminated disease:
(i) GI and pulmonary symptoms are often prominent. Ileus obstruction, massive GI bleeding, severe mal-absorption and peritonitis may occur.
(ii) Pulmonary symptoms: Dyspnea, hemoptysis and respiratory failure. Infiltrates may be seen on chest x-ray.
(iii) CNS: Parasitic meningitis, brain abscess and diffuse invasion of brain.
(iv) Liver infection: Cholestatic and granulomatous hepatitis.
5. Secondary bacterial infection of damaged mucosa may produce serious complications. High incidence of secondary gram negative sepsis, meningitis, pneumonia is probably due to disruption of bowel mucosa and carriage of bacteria on migrating larvae.
6. Blood Changes: A marked eosinphilia occurs during the invasive stage.
Laboratory Diagnosis
Stool Examination: Demonstration of typical Rhabditiform larvae under microscope in fresh stool is diagnostic.
Sputum Examination: Rhabditiform larvae rarely may be demonstrated in pulmonary infection.
Dudoneal Washing and jejuna biopsies may reveal larvae of Strongyloides stercolaris.
Prevention and Control Measures
- You should maintain proper sanitary disposal of faeces;
- Personal hygiene should be improved;
- Providing proper treatment of infected people;
- Making sanitary latrine and its proper use;
- Improved personal hygiene;
- Direct contamination of skin with soil should be prevented;
Treatment
To eliminate or prevent parasitic infection, preventive measures are required. To treat strogyloidiasis, antiparasitic drug is very effective. In this case, you should use a single dose of Ivermectin(Stromectol). Albendazole (Albenza) is also effective; it should be taken 10 days apart. You should also take thiabendazole (Tresaderm) as a dose of twice per day.
You might also read: Leishmania donovani: Morphology, Life Cycle, Leishmaniasis and Its Prevention
