The word ‘benthos’ comes from the Greek word ‘bathys’ which means depth. Benthos refers to some aquatic organisms that live above, inside, or near the bottom of a body of water. Such organisms live in flowing or stagnant water bodies and are also found in salt or freshwater habitats. The sedimental organisms at the bottom of the basin are called benthos.
Benthic animals can be divided into filter feeders (e.g. oysters) and deposit feeders (e.g., snails) (Ameen 1987). The biological productivity of aquatic systems depends on the bioavailability of plankton and benthos. Special knowledge on abundance, number of species and seasonal evolution is required for successful management of an aquatic ecosystem.
Benthos are an important part of the food chain, especially for fish. Many fish eat algae and bacteria. These are the steps in the food chain. Some eat benthos, leaves and other organic matter. Due to their abundance and location, they are known as middlemen in the aquatic food chain. Benthos plays an important role in maintaining natural energy flow and nutrient balance. When benthos die, they decompose and release nutrients that are reabsorbed by plants and other animals.
Benthic macroinvertebrates feed on algae and coarse organic matter (such as fallen leaves), fungi and bacteria, fine-suspended organic matter, and prey. Macroinvertebrates are part of the food chain that feeds many fish and other vertebrates in lakes and rivers. They live in a variety of water bodies, from small aquariums to the Mississippi River, the Great Lakes, and the ocean floor.
Some Benthos live a part of their life cycle in other habitats such as beach. Benthic macroinbertibrates are important fauna of freshwater, bay and marine aquatic ecosystems. These are usually invertebrates that can be filtered through a mesh size of 200-500 μm (Stickney 1984; Rosenberg and Resh 1993).
Types of Benthic Communities(Mackie, 1998)
The term phytobenthos is used to refer to the primary producer (e.g. different types of algae and aquatic plants). Zoobenthos, on the other hand, refers to all types of predators (such as benthic animals and protozoa). Benthic microflora (such as bacteria, fungi and many protozoa) form dissociative communities. They are involved in recycling energy and essential nutrients.
Benthos can be subdivided again based on size. Large benthic animals (seen without a microscope) are called macrozoobenthos or macroinvertebrates. They do not have vertebrae. They live on or at the bottom of special reservoirs as some parts of their life. These include snails, oysters, insects, amphipods, crayfish and the larvae of many aquatic insects (such as dragonflies, stoneflies, caddisflies, chironomid midges and blackflies). Microscopes (such as nematodes, astracodes) are needed to detect microbenthos.
Benthic organisms live in or above the sediment. Animals that live in sediments are called infauna. They primarily take in oxygen for their food and dissolution from sedimentary water. Some benthos live swallow sediments and use the food they contain and remove indigestible sand particles.
Benthic organisms that live on silt, rocks, tree fragments or vegetation are called epibenthos. The name suffix is added to identify the organism on which the substrate is attached.
Epifauna are creatures that are attached to animals (such as zebra mussels are often attached to snails, oysters, and crustaceans).
Organisms that live on rocks are called epilithic. Those who live on plants are called epiphytic. Benthos that live on mud or sand are called episemic creatures. Periphytons are microscopic organisms (most algae, bacteria, fungi, and molds) that live freely or attached to a submerged object. So both plankton and benthos phases live as periphyton. Sometimes it is difficult to determine which group is periphyton in lakes or rivers
Benthos is mostly classified on the basis of the area in which benthos lives. Lithoral and sub lithoral benthos can be identified by body appendages. They have clinging organs for attachment to the stems and leaves of plants. Profandal benthos are adapted duet to their filter feeding mechansms.
Generally, animals are missing here as there is no light in the profandal area. In fact, effective food groups in the profound region are worms, filter feeders such as snails and midge flies. Rarely, there is a fourth group benthos live here, called Abyssal Benthos. They live in very deep lakes (> 500 m) and most benthos species are blind.
Classification of Benthic Regions
Many limonologists have identified the area into three main areas from the deepest part of the bottom of a lake to the top of the beach. These regions are:
- 1. Littoral
- 2. Sublitoral and
- 3. Profoundal regions.
Moreover, if the lake is abnormally deep, a fourth layer called abyssal can be identified. This region extends from a depth of 600 meters to the maximum depth. The extent of these areas depends on the depth of the lake. Some lakes, for example, are so shallow that the whole bottom is part of the Litoral region.
(1) Littoral region: This region extends from the surface of the lake to the aquatic plants rooted below.
(2) Sublitoral region: This region extends from the area full of rooted aquatic plants to the upper level of hypolimonia.
(3) Profoundal zone: This zone includes the bottom of the lake surrounded by hypolimenion.
Distributionof Benthos
Benthos distribution are of two types,namely:
- Qualitative and
- Quantitative.
Qualitative Distribution
Biodiversity varies locally in the littoral region due to different conditions of the bottom, the action of the waves and other surrounding conditions. Different types of animals are more abundant in the littoral region than in the sublitoral and profound regions.
Biodiversity has been observed at depths of 0.5-3 m in the lake at different times. Different species live from the surface of different lakes up to a depth of 100 meters. However, the number of species varies from location to location.
In addition to microorganisms, there are many insects and mollusks in the littoral and sublitoral populations. More than 80% of the various Benthic groups belong to these two groups. Between these two groups there are more species of insects than different species of molluscs. As the depth of the lithoral region increases, the number of different types of benthic species decreases rapidly.
In special cases, the number of different species decreases at these levels of different lakes. However, in the temperate lakes of the first and second classes, the average of different species decreases. Within the first 18 m depth of these lakes, all species of sponges, Bryozoa, Platyhelminthes, snails, bivalves (except Spaeridae), nematodes, annelids and insects of almost all species are usually absent.
An ideal benthic population consists of a heterogeneous mixture of different animal groups. The number of animals varies in different places of the same lake. Baker (1918) divided the sedimentary material of Onida Lake, New York, into six groups, viz., Mud, sand, gravel, gravel, boulder, and sandy mud species. According to his research, 26 species of microscopic animals are found in all types of bottoms. 24 species were found in 3 types of soils, 16 species in 4 types of soils, 28 species in two types of soils and 8 species in one type of soils. In other cases, 114 species were found in muddy soils, 104 species in sandy soils, 83 species in strong muddy soils, 57 species in gravel soils, 63 species in boulder soils and 62 species in sandy soils.
Raushan (1930) studied in the 45 meter depth of Simoi lake of Canada. She recorded more than 35 species of protozoa, 2 species of turbellaria, small nematodes, 2 species of oligocoettes, 7 species of rotifers, 2 species of Copepoda, Nauplius larvae, few species of Ostracode, 1 species Gastrotricha, 1 species Tardigrada, and 3 species water mite.
Kabir and Naser (2009) conducted a study on Chandbil Baor and Harda Baor in Meherpur district, Bagladesh. They recorded a total of three groups of benthic animals, such as Chironomids, Oligocaetes and Moluscs.
Table: Different species of Benthos of dredged Harda baor and non-dredgedChandbil baor of Meherpur district, Bangladesh.
|
Recorded Benthos Taxa | |
|---|---|
|
Class: Insecta Order: Diptera Family: Chironomidae Genus: Chironomus Meigen Chironomus sp. Genus: Tanypus Meigen Tanypus sp. Family: Ceratopogonidae Genus: Ceratopogon Kettle and Tawson Ceratopogon sp. Class: Oligochaeta Family: Lumbriculidae Genus: Lumbriculus Grube Lumbriculus sp. Family: Naididae Genus: Nais MÜller Nais simplex Pignet Genus: Chaetogaster Von Baer Chaetogaster diastrophus Gruithuisen Chaetogaster crystalinus Vejdovsky Chaetogaster sp. Genus: Dero Oken Dero sp. Genus: Pristina Ehrenberg Pristina sp. Genus: Eiseniella Michaelsen Eiseniella sp. |
Genus: Stylaria Lamarck Stylaria sp. Genus:Branchiodrillus Michaelsen Branchiodrillus semperi Broune Branchiodrillus hortensis Stephenson Family:Aeolosomatidae Genus: Aeolosoma Ehrenberg Aeolosoma leidyi Cragin Aelosoma sp. Family: Tubificidae Genus: Tubifex Lamarck Tubifex tubifex MÜller Genus: Branchiura Beddard Branchiura sowerbyi Beddard Class: Gastropoda Family:Viviparidae Genus: Viviparus Montfort Viviperus bengalensis Lamarck Viviparus sp. Famiuly: Lymnaeidae Genus: Lymnea Lamarck Lymnea acuminata Lamarck Family: Ampullariidae Genus: Pila (Bolten) Roding Pila globosa Swainson Family: Hydrobidae Genus: Bithynia Leach Bithynia sp. |
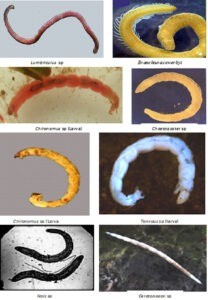
Image Showing Different Benthic Organisms
Quantitative Distribution
In shallow lakes: In the shallow lakes of the general type (class III), if the basin is not particularly diverse, the same productivity exists throughout the bottom. In most cases, such shallow lakes tend to be more productive. However, shallow conditions do not guarantee productivity and sometimes exceptions do occur.
The per unit area of the bottom region controls the growth of bottom animals.The amount of benthos produced at different depths of a very shallow lake is not the same, it is less than that of a deeper lake. The entire benthic region of a very shallow lake consists of the littoral region or littoral and sublitoral region. The extent of the benthos is similar to that of the lithoral region of the deep lake.

Image Showing Different Mollusks Species
In Deeper Inland Lakes: Inland lakes are divided into two parts from the point of view of quantitative distribution. (1) Lakes without chemical stratification in summer. (2) Summer chemical stratified lakes.This condition persists throughout most of the summer.
Nonstratified Deeper Lakes: Such lakes have no chemical stratification during the summer. As such lakes continue to circulate, a large supply of oxygen is maintained. The limited effects of stratification resulted in summer stagnation of hypoallergenesis. As a result, some benthic phases extend to the bottom of the basin with greater productivity.
Stratified deep lakes: Inland deep lakes have a stable stagnation in summer and an ideal hypolimonial state at the bottom. As a result, productivity is quantitatively lower than at the upper level. According to Eggleton (1931), a benthic concentrated area is observed in summer in the upper profundal and lower sublitoral regions of some Michigan lakes. As a result, this region has the largest population. In this region, the total population per unit area in the upper and lower basins decreases drastically.
Movements and Migration of Benthos
Benthos are thought to move on or below the surface in certain conditions. According to Moffett (Moffett, 1943), the lower fauna moves in flocks, driven by large waves.
As a result of the migration of the bottom, some changes can be noticed in the spread of the animals in the bottom. According to Moon (1935) (1) the littoral fauna of Lake Widermere can be observed to be constantly moving. (2) Such fauna is very sensitive to changes in the surface of the lake. (3) The more active phase of fauna is due to rapid movement in the newly flooded area of the beach. (4) When the height of the water increases by only 2.5 cm, the movement of these animals takes place.
Seasonal Changes in Benthos
Seasonal changes in benthos are rarely known. In all types of lakes and ponds of any depth, benthos undergoes seasonal changes. Seasonal changes occur in all types of benthic regions in very shallow lakes. Such changes are similar to the lithoral zone of deep lakes in the same region. The benevolent conditions of the Abyssinian region of these unchanging deep lakes remain the same throughout the year. The following is a list of the most common seasonal changes that occur in the benevolent fauna of an ideal second category temperate lake:
Litoral Region
Spring- (i) rapid growth of plants (ii) emergence of seasonal insects (iii) annual migration of Sailis larvae to the beach (iv) rapid reproduction of motionless animals (sponges and bryozoa) (iv) increase in population of various invertebrates, active growth and reproduction. (v) Snails migrate to shallow water for breeding.
Summer- (i) Plant growth reaches maximum level. (ii) Emergence of large insects (iii) shoreline migration of some may fly nymphs from plant areas (iii) Maximum abundance of different types of Chironomus larvae (iv) Major breeding season of many invertebrates.
Autumn- (i) Shallow aquatic vegetation is destroyed towards the end of autumn. (Ii) General extinction of fauna occurs due to the following reasons, namely: emergence of insects; Autumn storms; Sailis larvae, snails and others move downwards.
Winter– (i) Minimum growth of plants (ii) Decreased reproduction, growth and other physiological processes (iii) Many stages remain dormant, some animals become completely inactive. (iv) Some members go hibernation to deep bottom. (v) Sponge and bryozoa separate. (vi) Some summer plants inhabit the bottom region.
Sub-Litoral Region
Spring- (i) coming out of seasonal insects. (Ii) migration of Sailis larvae to the beach. (Iii) upward migration of snails and reproduction in shallow water. (iv) Reproduction through active growth and reproduction of various invertebrates. (v) Concentration of benthos in summer.
Summer– (i) coming out of insects which leads to decrease and increase in population. (ii) Subsequent offspring of insects are formed through egg hatching and larval development. (iii) No snails are present in shallow breeding ground. (iv) Different types of invertebrates continue to grow and reproduce. (v) The concentrated region of benthos is formed.
Autumn– (i) Downward migration of snails from summer breeding grounds. (ii) Adverse conditions cause the destruction of the benthos concentrated area and the downward movement of most of the population along the floor of the lake bottom. (iii) The amount of benthos decreases as a result of the coming out of insects. (iv) Downwards movements of Sailis larvae occur (v) Hatching of insect eggs occur in Autumn.
Winter-Population increases due to the following reasons in winter-: (i) Hatching occurs of eggs of insects generation of autumn. (ii) Downward movement occurs of snails, insects and other animals. (iii) Some insects do not appear. (iv) The insect larval growth period occurs in winter.
Profound Region
Spring- (i) At this time, the number of populations increases rapidly due to the entry of animals from the littoral and sub-littoral regions. (ii) Significant insects appear in late spring. (iii) The concentrated region of benthos is seen in summer at the beginning of the upper edge of this region.
Summer- (i) A higher density of benthos is formed on the upper edge of this region. (ii) Gradually the lowest levels of production are created.
Autumn- (i) Annual minimum production of summer is maintained at the beginning of autumn. (ii) There is a rapid increase in the population of benthos due to specific reasons, e.g.
- The depletion of water results in the influx of animals from the littoral and sub-littoral regions.
- Active reproduction of the genus Tubificidae occurs.
- Insects of autumn season appear.
- Insect eggs hatch in late summer.
Winter- (i) Tubificidae and Cerathra larvae appear towards the end of winter. (ii) Benthos occurs at their lowest level in winter. (iii) Abundance of insects do not occur.
Vertical Distribution of Profundal Bottom Fauna
Based on the results of the research, it is thought that most of the profundal bottom fauna exist in the surface above the bottom of the region. Lanz (1931) studied on different types of horizontal levels up to 24 cm depth. At greater depths in lakes in northern Germany, most of the fauna is found in the upper half of the Sampler.
Some differences have also been observed in the vertical distribution of different organisms. For example, the spread of benthos of the familyTubificidae can be observed upwards in the bottom mud. Chironomus larvae, on the other hand, extend from the surface of the mud to a depth of 20 cm or more. Another study found that macroscopic organisms are similarly widespread.
Classification of Lakes Based on Bottom Fauna(Benthos)
More or less 3 decades ago, some European limonologists classified lakes based on Benthic fauna. The bottom fauna of different types of lakes vary. Such variations in fauna are, in most cases, varied and complex. Excess of certain species or species groups are considered to be the identifiers of several types of lakes. Deevey classified Lake Connecticut in 1941 as follows:
- Lake Chironomus
- Mesotrophic Lake Chironomus
- Lake Tanytarsus
- Lake Trissocladius
- Non-stratified lake.
To date, no reservoirs have been classified based on the availability of benthic fauna in Bangladesh (Rahman and Das 2001). However, Khan et al. (1996) conducted a study on Kaptai Lake and mentioned the tropic level of the lake. According to them, Kaptai Lake is a feature between oligotropic and mesotropic. Thieneman (1925) divided the lake into three classes based on the availability of benthic fauna at the bottom of the lake.
Kabir and Naser (2009) conducted a study on Chandbil Baor and Harda Baor in Meherpur district, Bangladesh. They recorded the benthic animals per square meters as 2519 in Chandbill baor and 446 in Harda baor. Based on these results, they termed the two baors as high productivity and low productivity, respectively.
Abundance of benthic animals per square meter in Chandbil and Harda Baor of Meherpur District (Bangladesh) are shown in the following table (Kabir and Naser,2009):
| Species | Chandbill Baor | Harda Baor | ||||||
| Chironomids | Range | Total | Mean | SE(±) | Range | Total | Mean | SE(±) |
| Chironomus sp. | 222-4311 | 19466 | 1622 | ± 430.00 | 0-400 | 1510 | 126 | ± 31.34 |
| Tanypus sp. | 0-133 | 666 | 56 | ± 13.54 | 0-133 | 399 | 33 | ± 13.52 |
| Ceratopogon sp. | 0-711 | 1911 | 159 | ± 71.84 | 0-0 | 0 | 0 | 0 |
| Total Chironomids | 222-5111 | 22044 | 1837 | ± 469.17 | 0-489 | 1909 | 159 | ± 36.90 |
| Oligochaetes | ||||||||
| Lumbriculus spp. | 0-2400 | 3599 | 300 | ± 192.58 | 0-533 | 1021 | 85 | ± 46.22 |
| Tubifex tubifex | 0-267 | 933 | 78 | ± 26.34 | 0-44 | 44 | 4 | ± 3.66 |
| Branchiura sowerbyi | 0-44 | 88 | 7 | ± 4.94 | 0-44 | 44 | 4 | ± 3.66 |
| Nais simplex | 0-22 | 444 | 37 | ± 19.58 | 0-22 | 266 | 22 | ± 18.53 |
| Chaetogaster diastrophus | 0-267 | 356 | 30 | ± 22.80 | 0-44 | 44 | 4 | ± 3.66 |
| C. crystalinus | 0-133 | 266 | 22 | ± 12.80 | 0-0 | 0 | 0 | 0 |
| Chaetogaster spp. | 0-133 | 266 | 22 | ± 14.94 | 0-222 | 222 | 19 | ± 18.50 |
| Stylaria spp. | 0-44 | 44 | 4 | ± 3.66 | 0-0 | 0 | 0 | 0 |
| Branchiodrillus semperi | 0-89 | 177 | 15 | ± 8.34 | 0-178 | 488 | 41 | ± 19.33 |
| Branchiodrillus hortensis | 0-89 | 133 | 11 | ± 7.96 | 0-0 | 0 | 0 | 0 |
| Aeolosoma leidyi | 0-178 | 267 | 22 | ± 15.96 | 0-44 | 44 | 3 | ± 3.36 |
| Aelosoma spp. | 0-311 | 622 | 52 | ± 29.36 | 0-44 | 44 | 3 | ± 3.36 |
| Unidentified oligochaets | 0-133 | 443 | 37 | ± 13.19 | 0-400 | 1065 | 89 | ± 39.06 |
| Oligochaetes total | 177-2711 | 7638 | 637 | ± 203.92 | 0-976 | 3272 | 273 | ± 88.83 |
| Molluscs | ||||||||
| Viviperus bengalensis | 0-44 | 44 | 4 | ± 3.66 | 0-44 | 44 | 3 | ± 3.36 |
| Viviparus sp | 0-89 | 133 | 11 | ± 7.96 | 0-0 | 0 | 00 | 0 |
| Lymnea acuminata | 0-89 | 133 | 11 | ± 7.96 | 0-44 | 44 | 3 | ± 3.36 |
| Pila globosa | 0-44 | 88 | 7 | ± 4.94 | 0-44 | 44 | 3 | ± 3.36 |
| Bithinia spp. | 0-133 | 266 | 22 | ± 12.80 | 0-44 | 44 | 3 | ± 3.36 |
| Molluscs total | 0-178 | 664 | 55 | ± 20.51 | 0-44 | 176 | 15 | ± 6.25 |
| Total benthos | 667-5333 | 30345 | 2529 | ± 425.79 | 44-1154 | 5357 | 446 | ± 111.39 |
Role of Benthos in Aquaculture
Benthic macroinvertibrate has long been used as a biological indicator of water quality and an indicator of biological integrity from a management perspective (Gaufin 1973; Rosenberg and Resh 1993; Davis and Simon 1995). More recently, the location of habitats, the effects of hydrological changes, and the quality of water have been measured using benthic macroinvertebrates (Boonet al. 1992; Gore et al. 2001). It also plays an important role in changing or replacing ecological features (Merritt et al. 1996; 2002; 2008).
Ecologically benthic macroinvertebrates are primary consumers of plant material and predators of aquatic food traps. They use a higher trophic level in the aquatic food chain to convert primary production into biomass (Cummins et al. 2008).
A variety of aquatic ecosystems from freshwater to marine, they are the main consumers of the plants. Phytoplankton and decayed plant material from water, periphytic microalgae or vascular macrophytes or organic matter decomposed at the bottom are taken as food.
Macroinvertebrates are the main food component of advanced animal taxa. Moreover, people use them as food for recreational and commercial fisheries and wildlife species. They are also a major component of all types of biodiversity in the aquatic ecosystem. Taxes on all invertebrates are twice as high as on all types of vertebrates.
Benthic macroinvertebrates establish an important link between phytoplankton, zooplankton and fish stocks. Measurements of monthly benthos can measure the productivity of aquatic ecosystem. Knowledge of net production and assimilation of benthic species is essential for measuring trophic base for fish. In addition to being used as fish food, many benthic species play an important role in biological water purification.
Benthos also serve as a basic source of food for organisms in the ecosystem. This indicates the productivity of water (Latifa et al. 1997). These organisms provide the fish with the necessary food for fish farming in controlled water bodies. The seasonal variation in the range of benthic animals is very impressive. The abundance and amplitude of benthos vary with the change of seasons and the influence of physicochemical factors at different depths (Habib et al. 1984).
The Benthos are a dynamic community. They are important components of some trophic layers of the aquatic ecosystem. As herbivores and carnivores, they eat live algae, plants and invertebrates. On the other hand, it is the main food of many fish. As rotting substrate eater, they complete the recycling of organic matter and release nutrients from sediment by their activity.
The harmful effects of Benthos have also been reported. For example, large swarms of chironomids predominate among macrobenetic invertebrates, causing severe annoyance and causing significant economic damage to nearby homes and the local business and tourism industry (Ali 1996). Due to their importance, their production, species, spatial distribution, seasonal variation and various life history parameters such as growth rate, number of generations per year, emergence pattern of each individual species etc. are considered as significant topics of research
According to Jonasson (Jonasson, 1996), the amount, quality and quantity of hypoallergenic oxygen and water temperature are the major factors that affect the presence and mass of benign species in lakes and reservoirs.
