The unit of protein formation is the amino acid. 23 types of amino acids have been isolated from natural proteins. Amino Acid Requirements of Fish essential. In this case, 10 out of 23 are important for fish. Animals cannot make essential amino acids. So these have to be taken with food. The quality of food depends not only on the amount of nutrients, but also on the quality of the protein and the accumulation of amino acids in the food. For this, amino acids are divided into the following two groups, viz
- Essential amino acid
- Non-essential amino acid
1. Essential Amino Acid
Although there are more than 200 amino acids in nature, only 20 of them are found in all living things. Of the 20 amino acids, 10 are essential amino acids that fish cannot synthesize. Therefore, they must be supplied with food, so such amino acids are called essential amino acids. The following are the names of the 10 essential amino acids:
- Histidine
- Isoleucine
- Leucine
- Lysine
- Methionine
- Phenyl alanine
- Threonine
- Tryptophan
- Valine
- Arginine
Among the essential amino acids, lysine and methionine are the first limiting amino acids. Fish meal is made from vegetable protein (soybean) and it contains low levels of methionine. Therefore, excess methionine must be added to soybean-based foods for moderate growth and good health of fish. In addition, in fish farming, it is important for every fish species to have knowledge about the combination of protein and amino acid needs.
2. Non-essential Amino Acid
Certain amino acids are non-essential which the body can make through synthesis. These play a role in the management of various physiological processes in the body. Since such amino acids are produced in one’s body, it does not need to be supplied with food. Animal protein contains all the essential amino acids. Vegetable proteins, on the other hand, do not contain all the essential amino acids. The following are some of the non-essential amino acids:
- Alanine
- Aspartic acid
- Asparagine
- Cysteine
- Glutamic acid
- Glutamine
- Glycine
- Proline
- Selenocysteine
- Serine
- Taurine
- Tyrosine
Importance and Requirements of Amino Aacid in Fish
Amino acids play an important role in the body’s maintenance, growth, reproduction and cell tissue transplantation. Also some amino acids are quickly converted into glucose and provide energy to the brain and blood cells. Lack of essential amino acids causes the fish to lose growth, lose weight and lose its appetite. As a result, the body’s resistance to disease is reduced.
Alanine and aspartate are the main raw materials for fish glucose production and important sources of energy production. In addition, aspartate is essential for the synthesis of purine nucleotides in all cell types. Moreover, alanine is a more important nitrogen carrier for amino acid metabolism in the internal organs of fish (Mommsen et al. 1980). Aspartate and asparagine make up 10% of the amino acids in plant and animal proteins. Alanine can stimulate the diet of certain fish (Shamushaki et al. 2007). The addition of 5% pyruvate to the synthesis of Atlantic salmon and the synthesis of vitellogenin increases the level of alanine production without negative effects, resulting in an almost nitrogen-releasing environment (Olin et al. 1992). At present there is no information about adding aspartate or asparagine to fish diet. Alanine and aspartate are rapidly oxidized and are often used to maintain nitrogen balance due to lack of toxicity.
Arginine is found in large quantities in proteins (as peptide bond amino acids) and so it is needed in large quantities in fish feed. Citrulline is converted to arzinine by arginosaccinate synthase and lyase in the liver of elasmobranch and ureogenic teleost fish (Mommsen et al. 2001). In terrestrial animals, arginine is used for the synthesis of proteins, nitric oxides, urea, polyamines, proline, gutamate, creatine and agmatin (Wu and Morris 1998). Arginine also plays an important role in regulating endocrine glands and reproductive function (Jobgen et al. 2006; Yao et al. 2008).
18-20% of plant and animal proteins are leucine, isoleucine and valine. Leucine is said to be an active amino acid because it is an active effectors of certain rapamycin. It inhibits muscle protein synthesis and protein analysis in mammals (Nakashima et al. 2007).
Fish muscle and blood plasma contain large amounts of free amino acids, but gutamate and its decarboxylation products (glutamate, glutamine, and γ-aminobutyrate: GABA) are nerve stimulators that are present in the brain at high concentrations (1979). Moreover, glutamine is essential for the synthesis of purine and pyrimidine nucleotides in all cells. Glutamine also plays an important role in the production of renal ammonia in maintaining the acid-base balance in the body of fish. Glutamine and glutamate are 20% of the amino acids in plant and animal proteins, but they are completely dissociated in the gut in aquatic animals, such as in terrestrial mammals (Wu 1998). Thus, most of the glutamine and glutamate in blood plasma are synthesized from the amino acids and acetoglutrates attached to the skeletal muscle. Glutamate is used as a substrate in the synthesis of glutamine by ATP-dependent glutamine synthesis, whereas glutamine is hydrolyzed by phosphate-dependent glutamine to form glutamate (Anderson et al. 2002). Although there is an idea about this intracellular glutamine-glutamate cycle in mammals, but there is little information in fish.
Large amounts of glutamine synthetase are present in the brain, intestines, liver, muscles, gills, kidneys, and heart of fish. Cortisol (Vijayan et al.1996) or high environmental ammonia (Anderson et al. 2002) regulate liver proteins. Glutamine and glutamate are one of the most important sources of energy in fish, but tissue-based metabolism of these two amino acids has been determined in aquatic animals.
Excessive GABA in the diet interferes with the intake of food by Japanese flounders (Kim et al. 2003). On the other hand, the growth of Atlantic salmon and the addition of 5% α-chitoglutrate to the synthesis of vitellogenin almost reduced nitrogen emissions into the environment without negative effects (Olin et al. 1992). Providing gluten-rich foods to Asian carp increases fish weight, food intake, food conversion rate, and enzyme activity (Lin and Zhou 2006).
Integration of glycin and serine in the liver occurs through tetrahydrofalate dependent hydroxymethyltransferase. These two amino acids participate in glucose production, sulfur amino acid metabolism and fat digestion (Fang et al. 2002). Many fish also stimulate the intake of these two amino acids (Shamushaki et al. 2007). Glycin plays an important role in controlling the secretion of fins and shellfish (such as oysters).
Fish plasma albumin contains large amounts of histidine (Szebedinszky and Gilmour 2002). Fish muscle contains large amounts of such free amino acids or carnosine. Histidine plays a role in DNA and protein synthesis. Moreover, histidine acts as a source of energy when you are hungry. Histidine is a component of noncarbonate buffer that protects fish from starvation, abnormal swimming, and lactacidosis by altering pH values. Differences in non-carbonate buffer capacity have been observed in different fish species. This allows the fish to adapt to the environment for a long time. Interestingly, the concentration of intracellular histidine increased significantly before salmon was introduced during breeding (Mommsen et al. 1980). The metabolism of histidine in fish and its demand in food is regulated by a variety of environmental and endocrine regulators.
Lysine is one of the most important amino acids in commercial fish feed production, especially in the use of vegetable protein sources instead of fishmeal (Mai et al. 2006a). Lysine levels in the diet affect the health and growth of fish. In carnitine synthesis, lysine acts as a raw material that is required to transport long chain fatty acids from cytosol to oxidation in mitochondria. Adding carnitine to the diet leads to rapid physical growth and protects the fish from the toxins of ammonia and xenobiox. It increases fish reproduction by rapidly adapting to temperature changes and other environmental pressures (Harpaz 2005).
Phenylalanine is converted to tyrosine by tetrahydrobiopterin-based phenylalanine hydroxylase in the kidney and liver of fish.
Tyrosine is used as a raw material in the synthesis of important hormones such as thyroxine (T4), triiodothyronine, epinephrine, norepinephrine, dopamine and melanin and nerve stimulators. These elements have important controlling roles (Chang et al.2007; Yoo et al. 2000). Phenylalanine and tyrosine have a greater effect on the survival of fish in the natural environment, food intake, growth, color formation and disease prevention. Phenylalanine and tyrosine in the diet during fish conversion lead to rapid growth (Pinto et al. 2008). Furthermore, feeding thyroxine (T4) to carp, channel catfish, and flounder fish increases protein digestion, digestive enzyme activity, nutrient retention, growth rate, and feeding efficiency (Garg 2007).
Polyamines (putrescine, spermedin and spermin) are naturally occurring polysaccharides that are essential for cell growth and division. In mammals, it is synthesized from arginine derived from arginine or proline (Wu et al. 2008). Adding spermine to refined foods increases the activity of digestive enzymes and intestinal maturation, thereby increasing larval survival rates (e.g. European sea bass) (Pe´res et al.1997). Note that, high levels of polyamines are toxic to fish and have a negative effect on growth rate (Cowey and Cho 1992).
It is thought that proline is a non-essential amino acid in fish and stimulates the diet of fish. In mammals, proline is synthesized from arginine, ornithine, groutamine and groutmate (Wu and Morris 1998). Proline is currently considered a conditionally essential amino acid for fish larvae and mature stages.
Methionine is one of the most essential amino acids in some fish foods, especially high-protein vegetable protein sources such as soybean meal, almond meal, etc. (Mai et al. 2006b). Methionine and its derivative compounds can be made commercially through chemical processes. Methionine is usually found at adequate rates in the DL-phase. The natural isomer called L-methionine is quickly absorbed by animals and used efficiently.
Taurine is not associated with proteins but promotes fat digestion, anti-oxidation protection, cellular permeability, vision organs, nerves and muscular system development (Fang et al. 2002; Omura and Inagaki 2000). Fish meals and animal products contain large amounts of taurine (especially marine invertebrates) but are absent in plants. Providing taurine in the diet increases the intestinal permeability of cobia larvae which increases the efficiency of larval cultivation (Salze et al. 2008).
Threonine is a major component of small intestinal mucosa in fish. Tryptophan can be converted to serotonin (5-hydroxytryptamine; it is a neurotransmitter) and melatonin (resistant to corrosion) (Fang et al. 2002). Feeding with tryptophan prevents rainbow trout from providing aggressive behavior in their larvae (Hseu et al. 2003). It reduces cannibalism and food reluctance in grouper fish larvae (Ho¨glund et al. 2007) and plays a role in counteracting environmental stress by increasing cortisol levels (Lepage et al. 2003). Long-term use of cortisol in the diet has a negative effect on increased food intake, increased protein intake, and immunity (Vijayan et al. 1996).
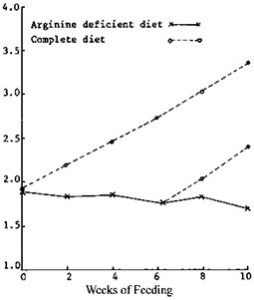
Figure-1: Growth of arginine deficient fish
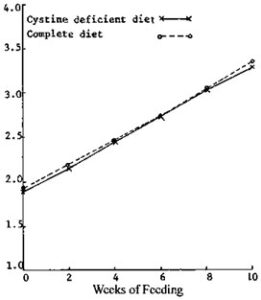
Figure-2: Growth pf Cistine deficiency fish
Table: Amino acid requirements of Cyprinus carpio
|
Amino acid |
1Amount of protein in food(%) |
2Amount of protein in food(%) |
3Amount of amino acid(mg/kg/day) |
|---|---|---|---|
|
Arginine |
4.3 |
4.4 |
506 |
|
Histidine |
2.1 |
1.5 |
145 |
|
Isoleucine |
2.5 |
2.6 |
145 |
|
Leucine |
3.3 |
4.8 |
429 |
|
Lysine |
5.7 |
6.0 |
458 |
|
Methionine |
2.1 |
1.8 |
105 |
|
Cystine |
5.2 |
0.9 |
– |
|
Phenylalanine |
3.4 |
3.4 |
254 |
|
Tyrosine |
2.6 |
2.3 |
190 |
|
Threonine |
3.9 |
3.4 |
213 |
|
Valine |
3.6 |
3.4 |
305 |
|
Tryptophan |
0.8 |
0.8 |
– |
Source: 1Nose(1979); 2Ogino(1980); 3Dabbrowski(1983)
Table: Amino acid requirements in carp fish
|
Amino acid |
Catla catla |
Labeo rohita |
Cirrhinus mrigala |
|---|---|---|---|
|
Arginine |
5.63 |
5.75 |
5.25 |
|
Histidine |
2.38 |
2.25 |
2.13 |
|
Isoleucine |
2.75 |
3.00 |
2.75 |
|
Leucine |
4.38 |
4.63 |
4.25 |
|
Lysine |
6.86 |
5.58 |
5.88 |
|
Methionine |
3.00 |
2.88 |
3.18 |
|
Phenylalanine |
4.50 |
4.00 |
4.00 |
|
Threonine |
4.50 |
4.28 |
4.13 |
|
Tryptophan |
1.03 |
1.13 |
1.08 |
|
Valine |
3.60 |
3.75 |
3.50 |
Source: Murthy (2002).
Deficiency Signs of Amino Acids in fish
|
Amino acids |
Deficiency symptoms |
|---|---|
|
Lysine |
|
|
Methionine |
|
|
Tryptophan |
|
|
Leucine |
|
|
Isoleucine |
|
|
Arginine |
|
|
Histidine |
|
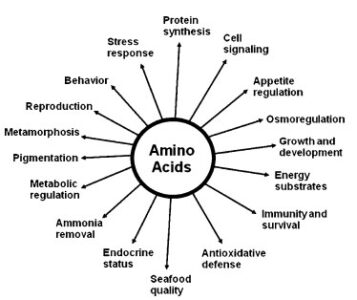
Role of Amino acid in growth, development and health of Fish
Table: The role of amino acids in the physiological function and metabolism of aquatic animals
|
Amino acids |
Manufactured Products |
Functions |
Species |
Referances |
|---|---|---|---|---|
|
Amino acids |
Different proteins |
Body composition, transport and control of various substances, disease prevention, transmission of signals to cells, supply of energy |
All aquatic animals |
Li et al.(2007) |
|
Alanine, Glutamil acid, serine |
Direct |
Appetite |
Many fish species |
Shamushaki et al.(2007) |
|
Arginine |
Nitric Oxide |
Destroying harmful microorganisms |
Channel catfish |
Buentello and Gatlin(1999) |
|
Arginine |
Nitric Oxide |
Neurological activity and physical development |
Telapia |
Bordieri et al. (2005) |
|
Arginine |
Nitric oxide |
Controlling the contraction and extension of muscle contraction, blood flow, regulating the viscous pressure in the gland and transmitting signals to cells |
Killifish |
Hyndman et al. (2006) |
|
Arginie and methionine |
Spermin |
Intestinal development during larval stage |
Seabass |
Peres et al. (1997) |
|
Arginine, Methionine. and glycine |
Creatine |
Acts as energy saver and antioxidant. |
Aretic charr |
Bystriansky(2007) |
|
Cistine, glutamine and Glycine |
Glutathione |
Antioxidants and transmitting signals to cells |
All type animals |
Wu et al.(2004) |
|
Glutamine and glycine |
Direct |
Removing ammonia |
Rainbow trout |
Anderson et al. (2002) |
|
Glutamine |
GABA* |
Helps in metamorphosis |
Abalon |
Morse et al. (1979) |
|
Glutamine |
GABA |
Controlling food intake |
Japanese flounders |
Kim et al. (2003) |
|
Glutamine |
Direct |
Achieving physical growth, food efficiency and intestinal development |
Carps |
Lin and Zhou (2006) |
|
Glutamine |
Direct |
Transmitting signals to cells, supplying energy |
Channel catfish |
Buentello and Gatlin (1999) |
|
Glutamine, glycine and asparagine |
Nucleotides |
Hereditary data storage, expression and biosynthesis |
Different fish species |
Li and Gatlin (2006) |
|
Glycine |
Direct |
Increasing in hepatic thyroxine (T4) and 5-monodyiodinease |
Rainbow trout |
Riley et al. (1996) |
|
Glycine |
Direct |
Controlling osmotic pressure |
Oyster |
Takeuchi (2007) |
|
Histidine |
direct and Carnosine |
Regulating pH change |
Salmon |
Mommsen et al. (1980) |
|
Leucine |
Hydroxy-β-methylbutyrate |
Increasing immunity, signaling cells |
Different fish species |
Li and Gatlin (2007) |
|
Lysine and methionine |
Carnitine |
Lipid transport in mitochondrial membrane |
Different fish species |
Harpaz (2005) |
|
Methionine |
Choline |
Cell membrane formation, nerve stimulation transport and betaine synthesis |
Different fish species |
Mai et al. (2006b) |
|
Proline |
Pyrroline-5-carboxylate |
Cell signal transmission, oxidation control |
Different fish species |
Phang et al. (2008) |
|
Proline |
Hydroxyproline |
Promoting physical growth, regulating collagen action |
Salmon |
Aksnes et al. (2008) |
|
Phenylalanine and tyrosine |
Thiroxine(T4) Tri-iodothironione(T3) |
Effects on metamorphosis |
Shol |
Pinto et al. (2008) |
|
Phenylalanine and tyrosine |
Thiroxine(T4) Tri-iodothironione(T3) |
Enhancing physical development |
Channel catfish |
Garg (2007) |
|
Phenylalanine and tyrosine |
Thiroxine(T4) Tri-iodothironione(T3) |
Helps in making pigment in the body |
Japanese flounder, rainbow trout |
Yoo et al. (2000) Boonanuntanasarn et al. (2004) |
|
Phenylalanine and tyrosine |
Epinephrine and norepinephrine |
Causing nerve stimulationImmunity control | Shrimp |
Chang et al. (2007) |
|
Tryptophan |
Serotonin |
Increase cortisol production, control behavior and food intake |
Rainbow trout |
Lepage et al. (2003) |
|
Tryptophan |
Melatonin |
Ovary development |
Salmon |
Amano et al. (2004) |
|
Taurine |
Direct |
Regulating osmotic pressure |
Carp |
Zhang et al. (2006) |
|
Taurine |
Direct |
Helps in adaptation |
Channel catfish |
Buentello and Gatlin (2002) |
|
Taurine |
Direct |
Intestinal development |
Cobia |
Salze et al. (2008) |
|
Taurine |
Direct |
Retinal development |
Glass eel |
Omura and Inagaki (2000) |
* GABA-Glutamate, glutamine, and γ-aminobutyrate
