Fish is one of the major sources of animal origin protein. Fisheries have a unique role in providing employment, foreign exchange earnings, and nutrition. About 65 to 70 percent of the protein we consume every day comes from fish. There are various species of fish belonging to the family Cyprinidae under the order Cypriniformes. These fish are known as carp fish. Fish of this type of carp are again two types, namely Major carp and Minor carp. Carp, which is larger with more economical value, is called the Indian Major Cup such as Rui, Catla, Mrigal carp, etc, whereas the smaller with less important economic value are called Minor Carp, such as Kalibaus, Bata, Ghonia, etc.
The Minor Carp fish carry fewer eggs than Major Carp. Apart from our indigenous or Indian carp, there are many different types of exotic carp in Bangladesh namely: Silver Carp, Grass Carp, Bighead Carp, Black Carp, Common Carp, etc. Carp fish eat different levels of water, so there is no competition for food and place.
Carp species are readily available, grow very quickly, and have higher immunity. Because of its delicious taste, it is also in demand in the market, so the cultivation of carp fish is increasing day by day in many countries.
Advantages of Carp Farming
- They eat foods from different levels of the water body.
- They are not rivals for each other food and place.
- They are not carnivorous.
- They have strong immunity against diseases.
- They grow rapidly and attain market size easily.
- Their larvae are available.
- Eats low-cost supplemental food.
- Delicious to eat and high demand in the market.
- Their economic value is high.
- Fry can be produced by artificial breeding.
Life history of Mrigal Carp (Cirrhinus cirrhosus)
Various scientists have described the life history of the Indian Major Carp. Ahmed (1944), Alikuni and Rao(1951), Alikuni (1956) described the early stages of minor and medium sized carp, such as Labeo gonius, L. bata and Cirrhinus reba. Khan (1925) studied on the development of Cirrhinus cirrhosus including several freshwater fish in Punjab.
Mookerjee et.al. (1944) and Mookerjee (1945, 1946) described the identifying characteristics of freshwater fish eggs and Indian common carp fries in Bengal. Mozumdar (1951) created a key for the identification of the eggs of the freshwater fish in Bengal. Mookerjee and Mzumdar(1946) described the life history of Labeo calbasu. Chacko and Kurian (1949) briefly described the early life history of Catla catla.
Systematic position of Mrigal Carp
- Phylum: Chordata
- Class: Actinopterygii
- Order: Cypriniformes
- Family: Cyprinidae
- Genus:Cirrhinus
- Species: Cirrhinus cirrhosus (Bloch, 1795)
Synonyms
Cyprinus cirrhosus (Bloch, 1795), Cirrhinus mrigala (Hamilton, 1822), Cirrhina blochii (Valenciennes, 1842), Cirrhina mrigala (Day, 1878), Mrigala buchanani (Bleeker, 1860), Cyprinus chaudhryi (Srivastava, 1968),
Physical Description
Mrigel fish is a well-known fish of the Family Cyprinidae, belonging to the order Cypriniformes. It is also called Migel or Mirka. In English, it is called Mrigal Carp, or Mrigal. Its scientific name is Cirrhinus cirrhosus. Its body is relatively cylindrical with bright appearance and the mouth is wide.
The lateral lines are complete and there are 40-43 scales present along the lateral line. The surface is gray and the sides are silver while ventro-lateral part is gray-black and the chest, pelvis, and anal fins are of orange color.
There are 16 soft rays present in the dorsal fins, 17 in the pectoral fins, 9 in the pelvic fins, and 8 in the anal fins. They are 25-30 cm tall and 330 grams in weight by the 1st year. In the second year, they are about 61 cm tall and about 2 kg in weight.
Habit and Habitat
They live on the bottom of the river. They are predominantly planktonic feeders but they also eat rotten plants or plant parts (Jhingran and Khan, 1979). In the larval stage, they mainly feed on zooplankton such as nauplii, rotifers, cladocerans, and copepods (Hora and Pillay, 1962).
According to Khan (1972) fry fish up to 100 mm in length, mainly feed on zooplankton, on the other hand with a length of 100-130 mm, fry feed on phytoplankton. Among the phytoplankton, the significant groups are Chlorophyceae, Myxophyceae, Bacillariophyceae, and Euglenophyceae.
According to Jhingran and Khan (1979), fish with lengths ranging from 300 mm to 560 mm receive up to 65–68% of rotten garbage. They also take fish meal, mustard oil cake, rice corn, etc. as supplementary food during cultivation in ponds.
They are mainly river fish but in Bangladesh, they are found all the freshwater water bodies such as a pond, beels, haor, baor(ox-bow lake), flooded paddy fields, and so on. Apart from Bangladesh, fish are also found in India, Pakistan.
Table: Dietary intake of fish at different life stages
| Open water: River, stream | Closed water: Pond, lake, etc. | |||||
| Food Items | Juveniles | Fingerlings | Adults | Juveniles | Fingerlings | Adults |
|
Green Algae |
5.1 |
5.9 |
14.2 |
3.0 |
6.2 |
10 |
|
Diatoms |
4.3 |
8.2 |
10.8 |
6.5 |
12.0 |
13.0 |
|
Blue-green Algae |
1.0 |
4.0 |
6.3 |
2.0 |
7.0 |
9.5 |
|
Desmids |
4.1 |
4.0 |
2.0 |
6.0 |
5.1 |
4.1 |
|
Phytoflagellates |
3.0 |
6.1 |
3.9 |
8.5 |
8.1 |
7.0 |
|
Algae Spores and Zygotes |
15. |
3.6 |
5.9 |
3.7 |
3.0 |
3.8 |
|
Microvegetation |
– |
2.1 |
1.0 |
1.2 |
1.0 |
– |
|
Decayed organic matter |
12.3 |
22.3 |
30.3 |
15.2 |
10.1 |
3.0 |
|
Protozoa |
6.3 |
4.3 |
1.3 |
6.0 |
5.0 |
2.5 |
|
Rotifers |
19.1 |
8.3 |
2.0 |
15.2 |
10.1 |
3.0 |
|
Crustaceans |
27.3 |
15.1 |
2.5 |
16.4 |
10.0 |
2.0 |
|
Sand and mud |
1.6 |
16.1 |
20.0 |
16.0 |
13.0 |
14 |
Data source : Khan (1972)
Reproduction
They are sexually mature within two years. During the monsoon or from May-July, they lay eggs in the aquatic vegetation in the shallow areas of the flooded river. During the breeding season, a mature female fish lays about one lac to eight lac eggs.
Embryonic development of Mrigal Carp
In the fertilized egg, the first cleavage occurs after 45 minutes of fertilization. As a result of this division, the blastodisk is divided into two distinct bastomomers. The same situation is observed in Catla and Labeo. The diameter of the fully developed egg is 4.5-5.5 mm and the average diameter is 5.0 mm. After the next 8-10 minutes, 4 cells stage are seen. In the next 10-15 minutes, 8 cells are seen.
About 1 and 1/2 hours after fertilization, 16 blastomeres are seen. In the early stages of fragmentation, the blastoderm holds a lens-shaped through some cells. As cell division progresses, its shape resembles that of a dome and spreads over the follicle. Within 3-4 hours, half of the yolk invasion is complete and within the next hour, the reproductive ring is spread over the entire yolk, so the inferior plug layer is obtained. In the next half an hour, the initial sightings embryos are developed.
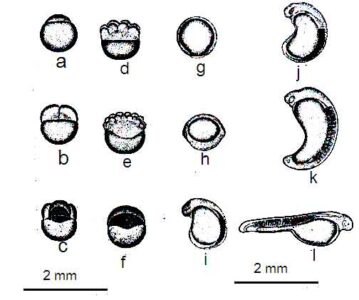
Fertilized Egg: (a) Newly formed bastodisk; (b) 2-cell phase;(c) 4-cell phase;(d) 8-cell phase;(e) 16-cell phase;(f) Morula phasev; (g)Yolk plug phase, embryo;(h) elongation stage of yolk cluster;(i) 6 somatic embryonic stage; (j)presence of optic cup; (k) extension of tail or tail from yolk cluster, formation of Kupfer`s cavity; (l) About 2 hours before hatching.
After about 7 hours of fertilization, the embryos can be separated into tail and head. The optic cup with 7 myotomes is seen in the embryo about 8 hours after fertilization. The head and tail part is not yet free from the yolk material. Then in half an hour, transparent parts (lenses) appear in the optic cup. The embryos, which are about 1/2 hours old, have a pair of autocysts and 19 myotomes.
Kupffer`s vesicles are visible at the end of the elongate yolk part. Such vesicles can be seen when the embryos are exposed to 14 myotomes. In this phase of development, a number of embryos begin to move slowly, and as the development progresses, the movement of the embryo continues to increase. Folding wings are seen on the dorsal and ventral side of the embryo at 12 hours of aged embryos.
Eggs begin to hatch after 16-18 hours after fertilization. This egg hatching process lasts for a few hours. The interval between the first and the last egg hatching is about 4 hours. At the time of hatching, most embryos have 42 somites.
Table showing various stages of larval life of Mrigal (Cirrhinus cirrhosus)
| Hatching/Hatching time (Hour) | 0 | 6 | 12 | 24 | 36 | 48 |
| Average total length (mm) |
4.20 |
4.5 |
5.0 |
6.21 |
6.70 |
6.84 |
|
Range(mm) |
4.04-4.32 |
4.18-4.80 |
4.89-5.06 |
6.02-6.49 |
5.96-7.47 |
4.79-7.55 |
|
Length of the yolk sac(mm) |
2.90 |
3.00 |
3.00 |
3.24 |
2.50 |
2.00 |
|
Maximum height of yolk sac (mm) |
0.90 |
0.90 |
0.80 |
0.54 |
0.30 |
0.25 |
|
Body height along the pectoral fins (mm) |
1.20 |
1.20 |
1.17 |
1.23 |
1.10 |
1.04 |
|
Number of pre-anal myotomes |
28 |
28 |
28 |
28 |
28 |
28 |
|
Number of post-anal myotomes |
14 |
1414 |
14 |
14 |
14 |
14 |
|
Eye diameter (mm) |
0.18 |
0.20 |
0.27 |
0.27 |
0.40 |
0.40 |
|
Eye color |
Colorless |
Colorless |
The center is black |
In some cases completely black. The center contains pigment. |
The center is dark black, completely covered by chromatophores. |
The center is dark black, completely covered by chromatophores. |
|
Length to the rear end of the notochord (mm) |
4.05 |
4.35 |
4.80 |
5.85 |
6.30 |
6.60 |
|
The direction of movement |
The bottom of the dwelling. |
If there is light, seldom vertical movement can be noticed |
Slow-moving, upward movement, rarely comes to the surface of the water. |
Runs at a somewhat intense speed; some show upward and some show zigzag movements. |
Some show horizontal movement. |
Horizontal movement and moving at high speed. |
|
Pectoral fin |
Absent |
Absent |
Absent |
Present |
Present |
Present |
Larval development of Mrigal (Cirrhinus cirrhosus)
Hatchlings
Their bodies are transparent, with no chromatophore. The face is not formed. The slender part is longer than the bulbous part of the dumble yolk. Eyes and otocysts are clearly visible. Moreover, the anal pore exists as a shaft where the yolk sac ends.

6 hours after hatching
There is a small dorsal shaft just behind the highest width of the yolk sacs. The notochord curves upwards from its apex. The back edge of the tail is evenly convex.
12 hours after hatching
The central pigment area of the eye is somewhat black and its border is colorless. Buccal invagination can be detected. The front of the egg yolk is globular. Pectoral fins develop poorly.
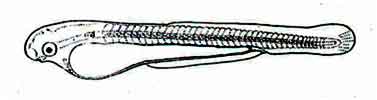
Fig: Larva of Mrigal: 12 hours after hatching of eggs
24 hours after hatching
The mouth is clearly visible. The eyes are more black pigmented. The anal groove is obvious. From the top of the notochord to the tail fin region lined spots exist. The slender edges of the yolk are sharp.
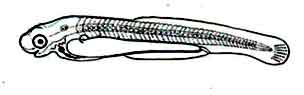
Fig: Larva of Mrigal: 24 hours after hatching of eggs
36 hours after hatching
Black chromatophores exist from the upper region of the yolk sac to the top edge of the notochord. However, there are few such chromatophores in the cranial region.
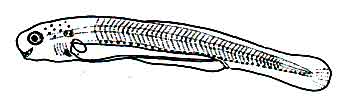
Fig: Larva of Mrigal: 36 hours after hatching of eggs
48 hours after hatching
Operculum covers the gill arches. The mouth is exposed. There is a dorsal groove in the oval region of the yolk along the upper side of the swimm bladder. At this stage the yolk sacis significantly reduced. The yolk sac is seen less than half the length of the embryo. The front edge of the yolk sac is straight.

Fig: Larva of Mrigal: 48 hours after hatching of eggs
72 hours after hatching
During this time the larval length is 7.20 mm. The dorsal region of the hatchling shows yellow. Clear eyes are present in these larvae. Two lateral rows of black chromatophores are seen along the body. Several prominent chromatophores are located in the abdomen and tail region behind the notochord. A group of such chromatophores also exist on swim bladder. The center of the eye is black. Throughout the lower half of the ventral side of the swim bladder, the embryonic fin buds originate. However, the dorsal fin buds originate from the posterior fossa. Only a straight row of chromatophores can be seen in the tail fin. In this situation, the lower part of the fetus, egg youlk is clearly visible.

Fig: Larva of Mrigal: 72 hours after hatching of eggs
Post-larval development
96 hours after hatching
At this time, the length of the young fisg is 7.34 mm. The youlk sac is completely absorbed. Dark black chromatophores are seen on the head. A few such chromatophores are also observed on the dorsal fins buds. The dorso-lateral surface of the young fish bears yellow color. A black pigmentation is seen in a semi-circular area below the anterior part of the notochord. The tail fin rays start to form. The pelvic fins become apparent; the dorsal fins begin to separate from the folds of the embryonic fins. The lips are thin at this time.
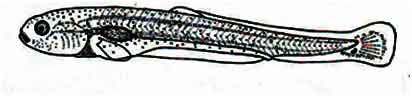
Fig: Post-larva of Mrigal: 96 hours after hatching of eggs
5th day after hatching
At this stage the length of the fingerling is 9.0 mm. The notochord curves upwards. The tail fin rays are more pronounced. The black chromatophores form a triangular spot in the peduncle region of tail. The chromatophores of the head are dark black.
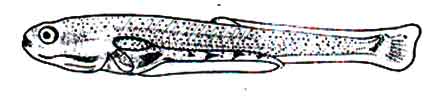
Fig: Post-larva of Mrigal: 5 days after hatching of eggs
6th day after hatching
At this time the length of the fish is 11.5 mm. 8 rays can be seen in the dorsal fin. A cluster of black chromatophores is seen on the 1st dorsal fin ray and the rays begin to branch out. Star-shaped black chromatophores are seen on the dorsal part of the head and body. The region from behind the eye to the caudal peduncle, the dorsal half of the body appears yellowish-green. The swimbladder is divided into two parts and the front part tends to be wider. This region is filled with black chromatophores. About 22 distinct rays can be seen on the caudal fin. Two black crescent-shaped marks are observed on the caudal peduncle. The fins of the anal fins are very weak. The ratio of the total length and the length of the base of the dorsal fin is 7 : 1.
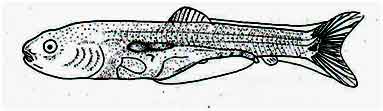
Fig: Post-larva of Mrigal: 6 days after hatching of eggs
7th day after hatching
At this time the larvae are 13.0 mm in length. 4 rays can be seen in the pelvic fins. The membranous embryonic pelvic fins begin to move from the abdomen to near the anus. The anal fins have 16 branched fin rays. 14 specific fin rays can be seen on the dorsal fin. Yellow pigmentation is seen at the base of the dorsal fin. A few black chromatophores are seen on the anterior part of the dorsal fin. The caudal fin has a membranous relationship with the body. This relationship is more pronounced on the dorsal side.
Under the microscope, there are two unclear semicircular disc-shaped markings below the tail fin that terminate the spinal cord. At this time 22 caudal fin rays are visible. A black chromatophore cluster on the front forms a triangle-like structure. The yellow pigment can be seen halfway from the base of the caudal fin. The swim bladder is covered by black chromatophores. From the tip of the caudal fin membrane, the caudal fin rays begin which is covered by yellow pigmentation and this pigmentation is denser on the dorsal side. At this time, the ratio of the total length and the base of the length of the dorsal fin is 6.6 : 1.
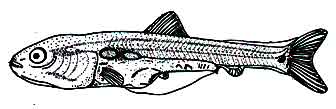
Fig: Post-larva of Mrigal: 7 days after hatching of eggs
8th day after hatching
At this time the length of the larva is 13.6 mm. 24 fin rays can be seen in the caudal fin. The fin rays appear yellowish up to half the length of the rays. The dorsal fin contains 16 fin rays. There are 7 fins rays in the anal fins. Black chromatophores are seen at the base of half of the tip of anal fin. Embryonic folds can be seen from the end of the abdominal region at the anal region.
The pelvic fins have 4 rays. Black chromatophores of different shapes are scattered all over the body. The chromatophores of the head and dorsal side are more pronounced due to their dark black and star-shaped. The lips are thin. The fish is golden yellow in color. The ratio of the total length and the length of the base of the dorsal fin is 6 : 1.
10th day after hatching
At this time the length of the fish is 15.6 mm. The dorsal side of the body is yellowish in color. With the exception of the first two rays, the remaining 14 dorsal fins are branched (Fig. 14C). One –third of the base of the fin rays is covered with yellow pigment. 26 fin rays can be seen in the caudal fin. The lateral line is visible as a dotted line. The dorsal part of the body is dark yellow, the rest is light yellow. 7 fin rays can be seen in both anal and pelvic fins. The ratio of the total length and the length of the base of the dorsal fin is 6: 1.
Fig: Post-larva of Mrigal: 10 days after hatching of eggs
12th day after hatching
At this time the length of the fish is 20.5 mm. The body is more pigmented than before. The entire edge of the dorsal fin looks black, especially in the dorsal half due to the dark chromatophore. Half of the caudal fin rays are covered by a dark yellow pigment. Pelvic fins have 9 fin rays; all are branched except the 1st fins ray.
The anal fins do not start from behind the anal opening. The first 3 rays of the anal fins are unbranched and the remaining 5 rays are branched. No barbell is observed at this time. The number of caudal fin rays is 36 which are covered with orange pigment. The tiny membranous part of the caudal fin is covered with black and yellow pigment. At this time the ratio of the total length and the length of the base of the dorsal fin is 6.5: 1.
15th day after hatching
At this time the length of the fish is 27.0 mm. The dorsal half of the body above the lateral line organ contains more black pigment. If the length of the fry is 24 mm, scales can be seen in the posterior region of operculum. The entire edges of the fins are covered with pigment. About 3/4 parts of fin length is black under the naked eye.
The fins are covered with a yellow pigment. Moreover, a few orange dots can be seen in it. The chromatophores are clearly arranged as a thick continuous band along the fins. Under the naked eye they look like black dots. The caudal fins have 16 rays. The half of the base of the caudal fin rays is orange in color and the edges of the fins are covered by black pigment. No barbells are observed at this time. The ratio of the total length of the body and the length of the base of the dorsal fin is 6.6 : 1.

Fig: Post-larva of Mrigal: 15 days after hatching of eggs
18th day after hatching
At this time the length of the fish is 28.0 mm. The fry are yellowish golden in color. Two black bands can be seen from operculum to the black caudal fin spot. One band is along the lateral line and the other band is slightly above the lateral line. The upper band is visible under the naked eye.
Above the lateral line, there are black spots on a dark yellow background. The lower part has less black chromatophores than the upper part of the lateral line organ. The body is completely covered by scales. The caudal fin has 34 rays. One-third of the base of the tail fin is yellow with orange pigment spots on it. One-thrird of the front of the tail is relatively colorless. The slightly diamond-shaped black dot is located on the caudal peduncle which can be seen under the naked eye. However, under the microscope, it looks like a spade.
20th day after hatching
At this time the length of the fish is 31.5 mm. No barbells are observed. Under the microscope, one-third of the 16 rays of the dorsal fin is covered with yellow-orange pigment. The pelvic fins are colorless. Slightly diamond-shaped black spots exist over almost the entire width on the caudal peduncle. These spots do not reach the edges. U-shaped formation is not seen on the dorsal margin of the caudal fin.
Orange pigments are spread on the edges of the caudal fin. The body is white below the lateral line but the upper part of the lateral line is yellowish and covered by black chromatophore. The margin of the scales are black.
25th after hatching
At this stage, the length of the fingerling is 39 mm. The body is silvery with a greenish hue. There are some scales with a thin black border. The underside of the tail fin is slightly reddish in color. Orange pigments are scattered up to the tip of the caudal fins. The upper lobe of the tail fin is slightly longer than the lower lobe.
Economic importance of Mrigal Carp
Mriigel fish is very tasty and nutritious. There is a huge demand in Bangladesh as food. Mrigel fish, along with other major carp, provide 60% of total exploitation from freshwater fish in Bangladesh. Every 100 grams of fish have 19.5 grams of protein, 0.8 grams of fat, 108 kilo calories of food energy, 350 mg calcium, 250 mg of phosphorus, and 1.1 mg of iron.
Apart from this, fishes also contain folic acid, niacin, and choline. Miguel fish have a lot of calcium and phosphorus, which contributes to bone and tooth formation. Phosphorous also removes knee pain and gout. Folic acid and iron-rich in fish help to increase blood.
References
Chakraborty, R.D. and Murty, R.S.V. 1972. Life history of Indian major carps, Cirrhina mrigala (Hamilton), Catla catla (Hamilton), and Labeo rohita(Hamilton). Journal of Indian Fisheries Society of India. 4:132-161.
Kabir, A.K.M. N. and Mia, Mohiuddin.2018. Fisheries Biology(in Bengali). 2nd edition. ATM Publications, 38/3, Banglabazar, Dhaka. pp. 623.
Rahman A. K. A. 2005. Freshwater Fishes of Bangladesh. 2nd edition. Zool. Soc. Bangladesh. pp. 394.
Shafi, M. and Quddus, M. M. A. 2003. Bangladeshher Matshya Sampad (in Bangali). Kabir Publications, 38/3, Banglabazar, Dhaka. pp. 345.


