The word ‘eutrophication’ actually comes from a Greek word meaning excessive feeding. When this process starts in a reservoir, it faces terrible consequences. Excessive feeding means that the basic substances that fall into the reservoir cannot be controlled and if this condition continues, the reservoir eventually becomes eutrophic and becomes a dead ecosystem. According to the Organization for Economic Co-operation and Development (OECD), eutrophication is the accumulation of excessive amounts of nutrients in a body of water, resulting in a number of unwanted symptomatic changes. Abnormal production of algae and other aquatic macrophages, for example, degrades water quality, taste and odor, and eventually kills fish and aquatic animals.
Eutrophication is a process of increasing the nutrient content of water which accelerates the growth of algae and higher plants. This process is influenced by external and internal sources of nutrients. Nutrients come from known or unknown scattered sources. Phosphorus is released from sediment in the form of particles. This source is considered as the main source of nutrients in water. Nutrients play a major role in eutrophication as a result of human activity in freshwater environments. This causes algae blooms. Cultural eutrophication in marine and Gulf systems increases the amount of nitrogen and phosphorus. However, in this case, the amount of silica does not increase. This increases the predominance of cyanobacteria and dinoflagellates from diatoms or chrysophytes. This process creates algae blooms in freshwater and marine ecosystems. As a result, toxins are produced based on the existing algae species. Significant amounts of toxins are produced that can have detrimental effects on human health in the ecological environment.
You might also read: Secchi Disc : General Description and Uses
Freshwater cyanobacteria produce hepatotoxins. Freshwater cyanobacterial species can also produce neurotoxins, anatoxins, and succitoxins. Marine dianoflagellates produce a variety of toxins, especially paralytic shellfish toxins, diarrheal shellfish toxins, and ciguatoxins. These algae cause problems in water purification and have a detrimental effect on the beauty of the marine environment. There are many methods used to control eutrophication or algae bloom. Integrated measures are taken to reduce the intrusion of nutrients into both freshwater and marine aquatic ecosystems. This includes strategies that reduce the readily available organic nutrients, especially phosphorus.
Eutrophication is a process that occurs all over the world. This process adds additional nutrients to various reservoirs such as lakes, rivers, creeks and oceans, resulting in changes in the initial production and species numbers in the community. This natural eutrophication process has been going on for many ages, mainly on the geological time scale. The cause of the Industrial Revolution is the addition of nutrients by humans to various water bodies. This type of eutrophication is called cultural eutrophication. This has a variety of detrimental effects on the ecosystem. Moreover, cultural eutrophication has a detrimental effect on human society through recreational opportunities and depletion of seafood, drinking water problems and phytoplankton poisoning of drinking water and seafood. Many problems with eutrophication arise as a direct result of algal bloom production. Such algal blooms cause poisoning or drastic changes in the ecology of water bodies.
Eutrophication mainly increases the levels of essential nutrients in water such as phosphate, nitrate and silicate (Lee et al., 1980; Uhllmann, 1980).
Nutrients from many sources in the aquatic ecosystem, such as chemical fertilizers, road water, animal excreta, and organic debris (such as leaves), make this problem difficult to solve.
Due to all these nutrients, the abundance of plants (mainly algae) increases. When the amount of nutrients is excessive, the algae blooms cover the surface of all the water bodies. As a result, sunlight cannot enter the water. In this condition, there are two types of problems in water.
(1) As a result, photosynthesis from the surface to the bottom of the water is prevented, and the amount of oxygen in the water is greatly reduced.
(2) The transfer of oxygen from the air to the water in the reservoir is stopped.
As a result, oxygen and sunlight in the reservoir are severely reduced. As a result, fish and plants suffer. When fish and aquatic plants begin to die, the decaying bacteria use the remaining oxygen to break down the dead organic matter. As a result, the water body is covered with algae, and there is a lack of aquatic plants and oxygen.
Aquatic plants and oxygen are needed for a healthy aquatic ecosystem. It has been observed that 80% of the total pollution in India comes from household sources with phosphorus content of 8 and 10 mg / liter. In 1984, 2.8 kg of detergent was used per person per year in India. In 2008 it increased to 4 kg. However, the use of detergents in rural areas increases at a rate of 7-8% per annum. This condition is serious because high quality detergents contain about 35% sodium dipolyphosphate.
Increased use of phosphate-based detergents increases the number of aquatic plants and cyanobacteria. Recently, there has been a growing awareness among scientists and the general public about the problem of abnormal growth of aquatic plants in water bodies, especially in lakes. 4/5 of the world’s freshwater drinking comes from lakes. Abnormal growth of aquatic plants and algae in the lake poses serious health risks to humans and animals.
Water bodies are generally classified based on variations in nutritional status. Algal blooms can be observed in water bodies under different nutrient conditions depending on the location. In Australia, for example, algal blooms have been observed to be below 1000 micrograms / liter of total phosphorus and 1000 micrograms / liter of total nitrogen and more than 10000 micrograms / liter of total nitrogen. Low levels of these elements cause algal blooms in Australia.
In European waters, on the other hand, algal brooms are produced at higher nutrient levels. It causes eutrophication and algal bloom. The following paragraph of the Bible contains instructions on the phytoplankton bloom. The directive states that “all the water of the river will turn red and the fish will die and the river will become a stagnant water body.” So the Egyptians will have no water to drink ”(Exodus 7: 20-21).
A recent report found that 54% of Asia-Pacific lakes, 53% European, 2৮% African, 4৮% North American and 41% South American are eutrophic.
Features of Eutrification
Eutrophication is a natural and man-made process that increases the supply of plant nutrients in natural water, resulting in the growth of weeds and higher aquatic plants. If the eutrophic condition in the water body continues for a long time due to natural causes, the trophic condition in the water body increases rapidly due to industrialization.
When the trophic condition increases due to industrialization, it is called cultural eutrophication. The following figure shows the eutrophication process and defines the term trophic condition of a reservoir. In eutrophic conditions, the quality of water is destroyed and the nutrients in the water and sediment are enriched.
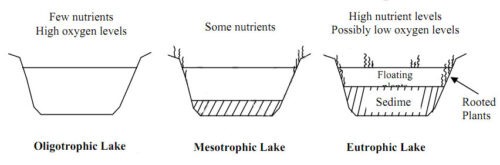
Eutrophication Process: Oligitrophic to Eutrophic Lake (Adapted from Henderson-Sellers B. and Markland H.R, 1987)
Eutrophication begins with nutrients coming from external and internal sources. Subsequent release of nitrogen fixation from the sediment results in reabsorption from parasites and mineralization of organic matter resulting in the release of dissolved nutrients.
The two main components controlling the growth of algae in temperate regions are phosphorus in particular as ortho phosphate and nitrogen as nitrate or ammonium salt. As the availability of these elements increases, aquatic ecosystems are lost and excessive growth of aquatic plants is achieved. However, this problem can be reversed through de-nitrification or oligotrophication by reducing nutrient depletion.
Oligotrophication can be achieved by physical removal of aquatic macrophages that receive nutrients in some reservoirs. However, continuous cycling in the system is not a suitable causation to eliminate eutrophication by controlling planktonic algae containing nutrients.
Eutrophic lakes have the following characteristics in different conditions:
1. The amount of dissolved oxygen in deep water decreases.
2. The concentration of nutrients in water tends to increase.
3. Biodegradable solids tend to grow.
4. The permeability of light tends to decrease.
5. The concentration of phosphorus in the sediment tends to increase.
6. A diatom-based algae population once evolved into a cyanobacteria (predominant) algae population.
Impacts of Eutrophication
- Abnormal growth of algae and aquatic plants leads to deterioration of water quality in reservoirs.
- Abnormal growth of algae and aquatic plants results in the taste and natural odor of drinking water.
- The bottom of the reservoir sinks after the death of the algae population. Their bacterial decomposition reduces the amount of oxygen at the bottom of the reservoir. As a result, the fish died.
- Due to lack of oxygen, the amount of iron and manganese in the water increases abnormally which causes problems in purifying drinking water.
- Abnormal growth of algae can cause filters to become ineffective in water purification plants.
- The use of such water has led to the spread of various diseases in tropical countries such as cystosimiasis, onchocerciasis and malaria. These diseases are the result of cultural eutrophication.
Eutrophications and Its Effects on Water Bodies
Criteria are widely used to determine the trophic condition of a reservoir. The idea that trophic theory is multifaceted and involves a variety of parameters is shown in the table below.
Table: Comparison of physical and biological properties of an oligotrophic and eutrophic lake
|
Parameter |
Oligotrophic Lake |
Eutrophic Lake |
|---|---|---|
|
The amount of green and bluish green algae |
Low |
High |
|
Daily migration of algae |
Sufficient amount |
Rare |
|
Characteristics of algae group |
Bacillariophyceac, e.g. Pinnularia, Cymbella; Chlorophyceae, e.g. Volvox; Chrysophyceae, e.g. Synura, Chromulina |
Cyanophyeae e.g. Microcystis, Nostoc |
|
Characteristics of the zooplankton group |
Presence of large sized species such as Daphnia, Cyclops |
Samll bodied species such as protozoans |
|
Characteristics of fish |
Different types of small-sized fin fish |
Different types of large sized fish |
|
The level of dissolved oxygen at the bottom |
High |
Low |
|
Density of nutrients in water |
Low |
High |
|
The concentration of nutrients in the sediment |
Low |
High |
|
Depth |
Deep |
Shallow |
|
Primary productivity |
Low |
High |
|
Species diversity |
Large variations |
Less |
|
Algae life |
Small variation |
Large amounts |
|
Plankton variations |
Large variations |
Less |
|
Bloom’s presence |
Rare |
Can be noticed in most cases |
Algae and oxygen are produced as a result of eutrophication and assimilation of readily available nutrients. This is why aquatic macrophytes can form blooms. The oxygen produced in this way is used by algae blooms and dead plants. Bacteria oxidize large plant components in large quantities. As a result, there is a lack of oxygen in the water. This causes the death of fish and other aquatic organisms. The oxygen used during digestion is called biochemical oxygen demand. Non-living bacteria produce hydrogen sulfide and methane gas at the bottom which degrades the quality of water.
Contaminants entering eutrophic reservoirs, such as nutrient contributions, can have detrimental effects on natural processes. Such herbicides and other contaminants may interfere with the self-purification process of aquatic macrophytes. Microscopic algae and aquatic macrophytes have different tolerance levels to man-made pollutants. Some toxic cyanobacteria are more resistant to some herbicides than macrophytes. In such cases, the effect of herbicides on the aquatic system increases the bloom of toxic cyanobacteria.
Influencers Causing Eutrophication
Eutrophication can be formed naturally in eutrophic environments. However, in most cases, major man-made changes, especially in land or surface water, can cause such conditions. With the increase in population in different countries of the world, the utilization of surface water has increased as the use of water and agriculture has increased. In Australia, for example, eutrophication has recently been identified as a major problem in maintaining water quality.
Eutrophication results in excessive growth of algae in the reservoir and severely degrades the quality of the reservoir. Australia has a low population density due to overcrowding, and many European countries have low levels of nutrients from man-made sources. Due to the concentration of nutrients in Australian water bodies, eutrophication is still occurring. Nutrients are added to the water flow from the drainage point to the specified source and from the scattered source (table). Moreover, reservoirs often contain nutrients that are released in certain conditions.
Table-2. Examples of Point souce, Diffuse source and In-stream sources of nutrients
|
Point souce |
Diffuse source |
In-stream sources |
|---|---|---|
|
Sewerage plant |
Strong tides flowing over rural lands |
Release of nutrients from sediment |
|
Dairy |
The water flowing over the city |
Due to break the bank of the river channel. |
|
Industrial waste |
Groundwater drainage |
Seasonal mixing of phosphorus rich surface water with deep layers of lakes or reservoirs |
|
Habitat of pigs |
Gaseous abnormalities |
– |
|
Irrigation drain |
Grazing |
– |
Water is collected in rivers, lakes and reservoirs through sedimentation through rocks, topsoil and bottom soils and organic matter. All these waters contain dissolved nutrients and other chemical elements which come from rocks and other elements. As a result, the quality of natural water varies with the change of space and time. Nutrients contain many types of primary and secondary nutrients. Experimental research has shown that phytoplankton take on a total of 16 different elements in different forms for survival. Phosphorus and nitrogen are the two main nutrients responsible for eutrophication.
Phosphorus plays a very important role in aquatic systems. A. Phosphorus is added to water in the form of colloid orthophosphate (PO43-) or organic phosphate. Colloidal precipitation accumulates and forms a layer of sediment at the bottom which usually releases phosphorus into the water column. The following figure shows the difference in eutrophication with the concentration of phosphorus.
However, if the level of orthophosphate in eutrophic reservoirs is less than 5 micro grams (µgm) per liter of water, then cyanobacteria bloom occurs in the water.
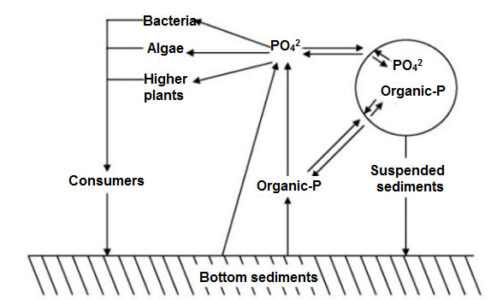
Image Showing Phosphorus Concentration
Phosphorus in most cases forms bonds with ferric hydroxide and in anoxic state the ferric ions decompose into ferrous ions and the weakening of the bonds causes the release of phosphorus in the water. Algae growth is achieved by taking this released phosphorus and this phenomenon is called creeping eutrophication. The release of phosphorus from the sediment involves some of the environmental regulating factors shown in the figure below.
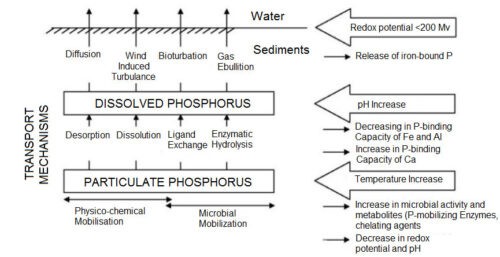
Figure: Some Environmental Regulatory Factors and Processes of Phosphorus Release from Sediment
Many years of research into the effects of phosphorus on algal blooms in lakes and artificial reservoirs have shown that phosphorus is a nutrient that acts as a controlling factor in most cases. Other physical factors such as light permeability and temperature can also play a role as a major controlling factor in low phosphorus concentrations. Nitrogen, on the other hand, can play a role as a controlling factor in tropical freshwater systems. Similarly, nitrogen restriction occurs in coastal waters before phosphorus restriction. Lack of nitrogen in the Gulf region reduces primary production.
Numerous studies have shown that the ratio of nitrogen to phosphorus is important for algae growth in some natural systems. The carbon / nitrogen / phosphorus ratio in phytoplankton is about 106: 16 : 1. The ratio of nitrogen to phosphorus in aquatic systems is usually 15-16: 1. The cellular components of phytoplankton are not stable. The maximum cellular nitrogen / phosphorus ratio is 7-87.
Nitrogen is an essential part of the cellular protoplasm of all organisms and is an integral part of all proteins and DNA. Nitrogen is the most important nutrient after phosphorus. Low availability nitrogen (N2) slows down algae growth, but some cyanobacteria, heterocysts and nonheterocysts, attach to atmospheric nitrogen to solve this problem. After the death of cyanobacterial cells, nitrogen compounds are released which creates new opportunities for nitrogen-bound organisms.
Before assimilation, nitrogen decomposes to form nitrate, then nitrate to form ammonia, which then forms proteins and other cellular compounds in a process related to photosynthesis. Excessive absorption of ammonia from nitrates has been observed in different types of marine and freshwater algae. Some algae may use purines as sources of organic nitrogen compounds such as urea, amino acids, and nitrogen. This type of condition can occur in more polluted environments. Aerobic or non-aerobic microorganisms in sediment cause biochemical conversion.
In the case of general management, there is a direct causal relationship between the amount of nitrogen and phosphorus. There is controversy over the growth rate of phytoplankton and the reduction of total bioavailability. The rate of absorption of dissolved nutrients from the reservoir and the regeneration of nutrients from algae are very important in a real ecosystem.
Concentrations of nutrients in very low doses indicate the depletion of nutrients from the water column which effectively reduces the growth of phytoplankton. A particular nutrient can become depleted or enriched if there is no balance between nutrient uptake and reproduction in soluble water. The turnover rate in dissolved inorganic phosphorus and dissolved nitrogen increases more rapidly from eutrophic reservoirs to oligotrophic reservoirs. The oligotrophic system is increasingly dependent on internal nutrient recycling.
Thermal stratification of a reservoir is a physical process that produces a biologically eutrophic system at lower concentrations of dissolved nutrients. Changes in temperature result in changes in the density of water. If there are two distinct layers in the same reservoir then thermal stratification of the reservoir occurs. As the surface of the water heats up in the spring, a thermocline layer is formed, and a warmer layer called epilimionion and a cooler dark oxygen-free bottom layer called hypolimionion are formed on top. The opposite happens in summer. During this time the surface of the reservoir is cold.
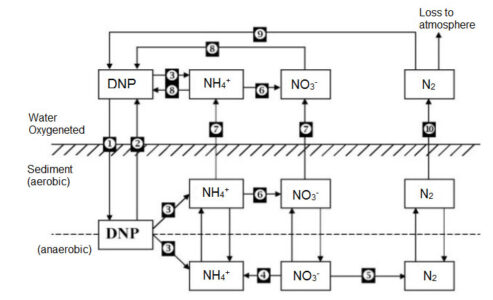
Figure: Major processes forming nitrogen cycle in sediment / sedimentation system [1-Sedimentation, 2-resuspension, 3-ammonification, 4-reduction, 5-denitrification, 6-nitrification, 7-diffution, 8-assimilation, 9-gas convection, 10-nitrogen fixation, DNP denotes nitrogen bound as organic forms such as nucleotides. ]
Remedies for Eutrophication
First we need to determine the nature of the eutrophication problem and the goals of the control program. Eutrophication problems include excessive growth of algae or macrophytes, decreased water clarity, decreased hypoallergenic oxygen, resulting in death of the fish. The regeneration of nutrients or the chemical composition of water, the quality of smell and taste is reduced. Drinking such water causes problems.
Water Recovery Strategies
Water is restored based on the purpose. In this case different types of methods are used in different parts of the world. The advantages and disadvantages of each method are mentioned below:
Diversion of Wastewater
This method is widely used in lake recovery. In this case, the water is removed from the lake and the replaced water is purified. Influence is emitted in low-lying areas in an ecosystem that is less sensitive to existing reservoirs. The impurities are removed from the effluent by mechanical purification. Diversion brings positive results if eutrophication is a major problem in a lake. In the case of many types of eutrophication, this problem is solved by digging canals at the outlet of the sea or lake. The most notable example of diversion is Lake Washington in Seattle. In 1980, wastewater was removed from the lake at the coastal Puget Sound. As a result, Lake Washington improved rapidly. Lakes can be restored quickly if diversions are made to bring in less nutrient-rich water from other sources.
Removal of Surface Sediment
A highly eutrophic lake can be restored by silt removal. This method is applied to small ecosystems with extensive care. Sediment has high nutrient concentration and many toxic elements especially trace metals. The exchange of sediments and water disrupts the restoration of the ecosystem. When the wastewater treatment process starts, the speed of these exchange processes increases. In most cases, phosphorus reacts with iron phosphate sulfide to produce iron sulfide through phosphorus release.
Uprooting and Removal of Macrophytes
Uprooting macrophyte root and removal methods are widely used in rivers and some artificial reservoirs. This method is mainly used in cases where macrophages are responsible for causing eutrophication. A comprehensive balance must always be maintained to assess the significance of the method with the nutrients coming into the reservoir. In all cases, plant fragments are collected. Moreover, spontaneous removal of nutrients from incoming effluent is also considered.
Coverage of Sediment by an Inert Material
Sediment is covered by an inert substance. According to this idea, the exchange of nutrients (possibly toxic substances) between silt and water can be prevented. Polythene, polypropylene, phosphorus screens or clay are used to cover the surface of the sediments. The use of this method is limited due to high cost.
Siphoning of Hypolimnetic Water From Reservoirs
In the case of artificial reservoirs or large ponds, it is much easier to apply this idea to reduce the causes of epileptic eutrophication. Phosphorus is not used in clustering or concentration as calcium hydroxide. It is considered to be the best ingredient for precipitation in wastewater and its action is pH dependent i.e. its action requires a pH value of 9.5 or higher. It cannot be said for sure that all bunches of phosphorus will sediment and phosphorus mixes with sediment. This method is not commonly used as phosphorus may be released from water at a later stage.
Water Circulation and Ventilation
Water flow can be used to break down the thermocline layer. As a result, no anaerobic region is formed. Thus phosphorus is not released from the sediment. Non-living conditions can be directly prevented by air conditioning in lakes and reservoirs. Ventilation is used to remove the anaerobic condition of highly polluted rivers and canals. The Danish lake Hald uses pure oxygen instead of air. Oxygen does not have lasting results from other techniques such as siphoning of hydrolimnetic water.
Hydrological Control
Hydrological regulations are widely used in flood control. Most recently, this method has been considered as a significant method of changing the ecology of lakes, reservoirs and wetlands. As the storage time of lakes and reservoirs decreases with the annual input of nutrients, the concentration of nutrients decreases as a result the eutrophication reduces. Wetland productivity is highly dependent on water depth. In this method, dams are constructed around the reservoir based on the depth of eutrophication control. As a result, excess nutrients cannot enter the reservoir from the outside, thus reducing the chances of eutrophication in the reservoir water. So it is easy to control the wetland ecosystem through this method.
Control of Chemical Fertilizers
Eutrophication can be controlled by controlling the intake of high levels of nutrients in reservoirs or lakes. Chemical fertilizers can be controlled in agriculture and forestry to reduce the wastage of nutrients in the environment. The use of nutrients by plants depends on a significant number of factors (such as temperature, soil moisture, plant growth rate). The presence of cyanophyte blooms greatly determines the N: P ratio in lake water. If the ratio of N: P is less than 5, 50% of the bloom forming material will be cyanophyte. If the ratio of N: P is less than 2, 100% cyanophyte bloom can be seen. The main source of phosphorus is contaminated water. The concentration of phosphorus in purified wastewater can be easily reduced by creating a chemical precipitation of 1 mg / liter and even 0.1 mg / liter of treated wastewater. All measures can be taken to prevent cyanophyte bloom from occurring, such as chemical fertilizer control, wastewater treatment and keeping the N: P ratio appropriate, i.e. above 7
Nutralization of Calcium Hydoxide
Calcium hydroxide is widely used to alleviate low pH values in rivers or canals where acid rain has a significant effect. Sweden spends 100 million every year to soothe acidic water in rivers, canals and lakes.
By Applying Algae Killer
Various types of copper salts such as copper sulphate is used in small lakes. Copper is now rarely used for general poisoning. Copper salts accumulate in silt and thus contaminate a lake over a long period of time. The effect of copper on algae varies from species to species. Blue-green algae are generally more sensitive to copper ions. Studies have shown that metabolism causes copper to accumulate in cells and to increase its concentration in animals with higher trophic levels (Mitsch, 1980).
Shoreline Vegetation
In this case trees are planted along the coastline.
Biomanipulation
If the phosphorus concentration in the lake is 50-150 mg / liter, biomanipulation method is used to restore the lake. If the phosphorus concentration is low at the beginning and continues to increase, grazing is able to maintain a low concentration of phytoplankton, which indicates a relatively low concentration of phytoplankton. Zooplankton grazing at a certain phosphorus concentration (about 120-150 mg / liter) is not able to control the phytoplankton concentration for a long time. The result is almost an increase in water turbidity and an increase in the abundance of plankton-eating fish which also preys heavily on zooplankton. There are two ecological structures in the dose of 50-150 mg / liter of phosphorus. This level of phosphorus can be maintained by removing plankton-eating fish and releasing carnivorous fish.
Some improvement of water quality is required to keep the phosphorus level at 150 µg / liter through biomanipulation. However, sooner or later the lake acquires an ecosystem structure with high phosphorus concentration. In that case biomanipulation is a relatively inexpensive and effective method of controlling phosphorus levels. Chinese grasscarp or white amur eats mainly submerged and small floating plants. They can survive well in warm water and cool water. Small fish eat plant material several times their body weight as food. To increase the body weight of the fish per 1 gram, it is necessary to eat 48 grams of Hydrilla plant. About 75 fish can eat one hectare of weeds in a pond.
The use of white amur in aquatic weed eradication in India is steadily increasing. It has been observed that 10,000 fingerlings of native Puntius pilchellas, (weighing 10-14 grams each) consume 25-50 kg of Limnea and Hydrilla plants daily. Thus they consume 9-18 tons of aquatic weeds every year. Other fish species such as Tilapia zilli, Tuilapia quinensis, Silver carp (Hypophthalmichthyes molitrix), Silver dollar fish (Metynnis argenteus), Common carp (Cyprinus capio), Goldfish (Carassiu auratus), etc play a great role to control aquatic weeds. .
